August 2018
Genetic ‘weather forecast’ could predict bowel cancer relapse
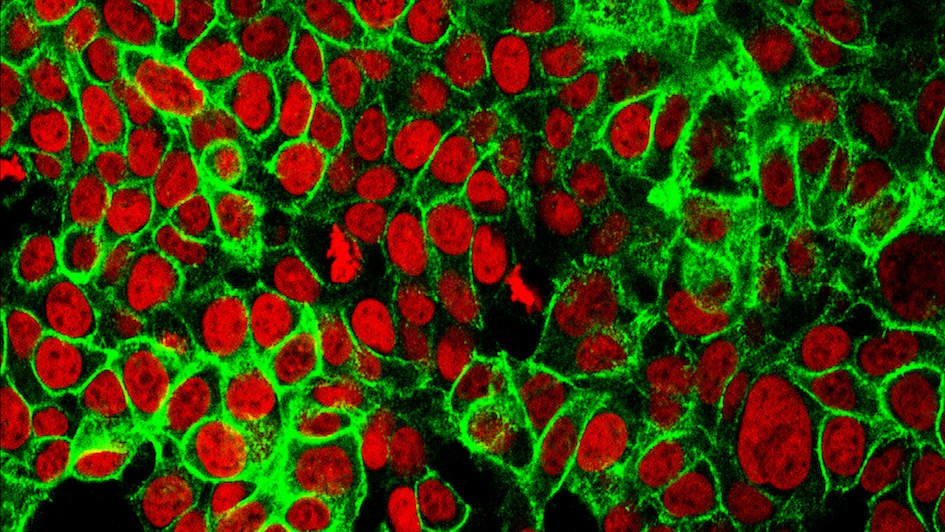
Image: Human colon cancer cells with the cell nuclei stained red and the protein E-cadherin stained green. Image credit: NCI Center for Cancer Research. Licence: Public domain.
ICR scientists have developed computer models that can track the evolution of cancer from tumour DNA in blood samples, to predict how cancer will respond to treatment like forecasting the weather.
Our researchers measured changes in tumour DNA in blood taken from patients with advanced bowel cancer, which could signal changes in the way tumours are growing and responding to treatment.
The model could predict when patients would stop responding to bowel cancer drugs and when their cancer would return, even before CT scans in the clinic.
Forecasting how tumours will change over time could help clinicians plan treatment better, or offer alternative therapies sooner.
The study, published in the prestigious journal Cancer Discovery, was led by Professor Andrea Sottoriva, Professor of Cancer Genomics & Evolution and Deputy Director of the ICR's Centre for Evolution and Cancer, and Professor Nicola Valeri, Professor of Gastrointestinal Oncology at the ICR.
Professor Sottoriva said: “Our computer model can predict how tumours will evolve and estimate how long it could take for cancer to return after treatment, like how computer models forecast the weather. Understanding how tumours evolve in response to treatment is key to delivering better care for patients.”
The study was supported by funders including Cancer Research UK, the NIHR Biomedical Research Centre at the ICR and The Royal Marsden NHS Foundation Trust, the Wellcome Trust and the European Union.
October 2018
Major trial shows targeted drug palbociclib extends breast cancer survival
An international clinical trial led by ICR researchers found that women taking a targeted drug, palbociclib, together with hormone therapy lived longer than those on hormone treatment alone.
The drug combination extended the lives of women with advanced, hormone-sensitive breast cancer by an average of seven months. These patients also saw a longer delay until the start of chemotherapy, which can have debilitating side effects.
The findings helped pave the way for palbociclib becoming available on the NHS for women whose cancer has progressed on prior hormone therapy.
The results of the trial were published in the highly prestigious New England Journal of Medicine.
The trial was led by Professor Nicholas Turner, Professor of Molecular Oncology at the ICR and Consultant Medical Oncologist at The Royal Marsden. He said:
“It’s incredibly rewarding that the benefits we had previously seen for palbociclib are now translating into such significant extensions in survival.
“This drug can offer women more precious time with their loved ones and because it is a targeted treatment it is much kinder than chemotherapy, and enables many women to carry on with their lives normally.”
The trial was funded by the manufacturer of palbociclib, Pfizer.
November 2018
Immunotherapy extends lives of patients with head and neck cancer
Image: Drugs for a clinical trial
The ICR’s researchers showed that a new immunotherapy could greatly extend the lives of people with advanced head and neck cancer, with some living for over three years.
They evaluated the drug – pembrolizumab – in a trial of nearly 500 patients with very advanced disease that had already spread and become resistant to chemotherapy.
Treatment options for these patients are extremely limited, and they are normally expected to survive for less than six months.
Overall, patients who received pembrolizumab experienced significant benefits – with 37 per cent surviving for a year or more, compared with only 26.5 per cent of those on standard care, consisting of chemotherapy or the targeted agent cetuximab.
Some 36 patients saw their cancer partially or completely disappear, and some were still cancer-free three years after first receiving pembrolizumab.
Study leader Professor Kevin Harrington, Professor of Biological Cancer Therapies at The ICR and Consultant at The Royal Marsden, said:
“I would like to see pembrolizumab approved for use in the clinic, so that people with metastatic head and neck cancer can be offered the chance of a longer life and improved quality of life.”
The trial, published in The Lancet, was funded by Merck & Co., Inc.
January 2019
New AI test identifies women with 'very high risk' ovarian cancer
-mucinous-ovarian-tumour-(photo-nephron)-carousel.jpg?sfvrsn=ed407e69_10)
Image: Intermediate magnification micrograph of an ovarian tumour. Credit: Michael Bonert. License: CC BY-SA 3.0
ICR scientists have created a new test that scans the shapes of tumour cells to pick out women with especially aggressive ovarian cancer, so treatment can be tailored to their needs.
They used artificial intelligence to look for clusters of cells within tumours with misshapen nuclei – the control centres within each cell.
In the study they found that having misshapen nuclei was an indication that the DNA of cancer cells had become unstable – a sign of aggressive cancer which could help doctors select the best treatment for each patient.
Cancers with misshapen cell nuclei had hidden weaknesses in their ability to repair DNA, which could make them susceptible to drugs called PARP inhibitors or platinum chemotherapy.
Study leader Dr Yinyin Yuan, who leads the Computational Pathology and Integrative Genomics team, said:
“Using this new test gives us a way of detecting tumours with hidden weaknesses in their ability to repair DNA that wouldn’t be identified through genetic testing. It could be used alongside gene testing to identify women who could benefit from alternative treatment options that target DNA repair defects, such as PARP inhibitors.”
The study was published in the journal Nature Communications and was funded by the ICR itself.
February 2019
Cell death triggers keep cancer at bay in healthy cells
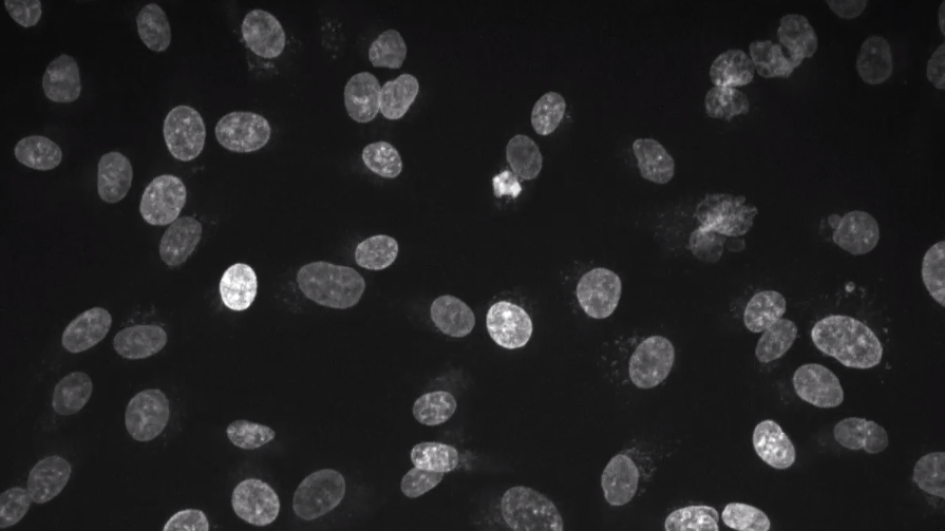
Image: Capture of live cell imaging of fibrosarcoma cells. Credit: Gianmaria Liccardi and colleagues, published in Molecular Cell. License: CC BY-4.0
Our researchers found that two proteins that play a key role in triggering cell death also help protect the genome and prevent cancer in healthy cells.
The proteins, RIPK1 and Casp8, are known for their role in triggering cell death, but our researchers found that they also help to coordinate cell division.
The two proteins help chromosomes line up during cell division, preventing healthy cells from becoming cancerous by stopping the genetic faults that trigger cancer. The researchers found that disrupting or deleting RIPK1 and Casp8 in cells and mice caused chromosomes to become misaligned, leading to genetic mutations.
As chromosome instability is a defining feature of cancer, understanding how these proteins work together could open up new ways to treat the disease.
Study leader Professor Pascal Meier, Head of the Cell Death and Immunity Team, said:
“Our study has shed light on the biological processes that help determine whether a cell is healthy or could become cancerous. Loss of function of these proteins is linked to a range of cancers and degenerative diseases, and learning more about them could lead us to new cancer treatments.”
The research was supported by Breast Cancer Now, the Medical Research Council and the NIHR Biomedical Research Centre at The Royal Marsden and the ICR, and was published in Molecular Cell.
March 2019
New cancer preventing role found for DNA cable tie

Image: DNA strand
ICR scientists discovered that a ring of proteins called cohesion, which holds our chromosomes together as they replicate, also plays a vital role in stopping DNA damage from leading to cancer.
Cohesin is made up of proteins that work together to hold strands of DNA in place like a cable tie and these proteins are often mutated in cancer cells. When cohesin is unable to protect DNA, researchers found that DNA is repaired with more mistakes – which can lead to cancer.
Many researchers had concluded that cohesin helps to prevent cancer by holding chromosomes together during cell division, ensuring that daughter cells inherit the right number of chromosomes.
However, Professor Jessica Downs’ team reasoned that cohesin must be involved in preventing cancer in other ways, because some cancers with mutations in their cohesin proteins do not have the wrong number of chromosomes.
Professor Jessica Downs said:
“Our research has uncovered a new function for cohesin, showing that its role in repressing transcription at DNA breaks helps to prevent genome instability, and furthering our understanding of this incredibly important tumour suppressor.”
The research, published in Molecular Cell, was funded by the Medical Research Council and Cancer Research UK.
July 2019
Cancer trades in sugar for fatty acids in order to spread around the body
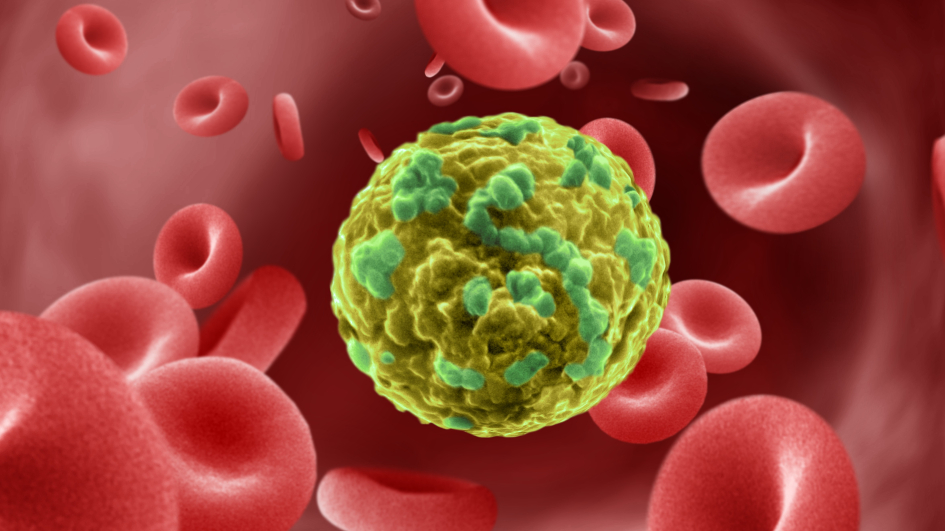
Image: Cancer cell migrating through blood vessel. Credit: Annie Cavanagh. License: CC BY-NC 4.0
ICR scientists uncovered a crucial change in cancer cells that allows them to spread around the body – by switching from sugar to fatty acids to fuel their growth.
Researchers found that high levels of the protein AKR1B10 help cancer cells adjust to new environments and form new tumours in other organs.
When cancer cells have high levels of AKR1B10, it reduces their dependency on sugar and increases their ability to use fatty acids as a fuel source instead.
Cells normally avoid using fatty acids for fuel because process produces toxic byproducts. High levels of AKR1B10 limit this toxicity, allowing the cancer cells to thrive in new areas in the body.
Professor Clare Isacke, Professor of Molecular Cell Biology in the Breast Cancer Now Research Centre at the ICR, said:
“Our study has shown the importance of cancer cells learning how to use different nutrients and energy sources in order to survive, thrive and spread.
“This research significantly improves our understanding of cancer cell metabolism and metastatic relapse and could lead to new avenues of exploration for treatments in future.”
This research, published in Nature Communications, was funded by Breast Cancer Now, working in partnership with Walk the Walk.
July 2019
Immunotherapy could work in treatment-resistant bowel cancers
Scientists at the ICR found that bowel cancer patients who had stopped responding to a widely used targeted drug could benefit from immunotherapy.
Bowel tumours which had initially responded to cetuximab before developing resistance became more visible to the immune system – potentially leaving them vulnerable to immunotherapies.
The new study, published in Cancer Cell, offered a detailed picture of the many different ways in which bowel cancers can evade cetuximab treatment either from the outset or by acquiring drug resistance.
The team have begun a phase II clinical trial to test the possible benefit of immunotherapy in patients who have stopped responding to cetuximab.
The research was led by Dr Marco Gerlinger, Team Leader in Translational Oncogenetics at the ICR, and Consultant Medical Oncologist at The Royal Marsden.
He said “Most bowel cancers are ‘immune deserts’ – so it’s enormously exciting to see that the targeted drug, cetuximab, attracts immune cells into these tumours.
“I’m eager to see if immunotherapy can unleash the immune cells and shrink these bowel tumours.”
The study was largely funded by Cancer Research UK, with additional support from the NIHR Biomedical Research Centre at the ICR and The Royal Marsden.
October 2019
New ‘evolution-busting’ cancer drug
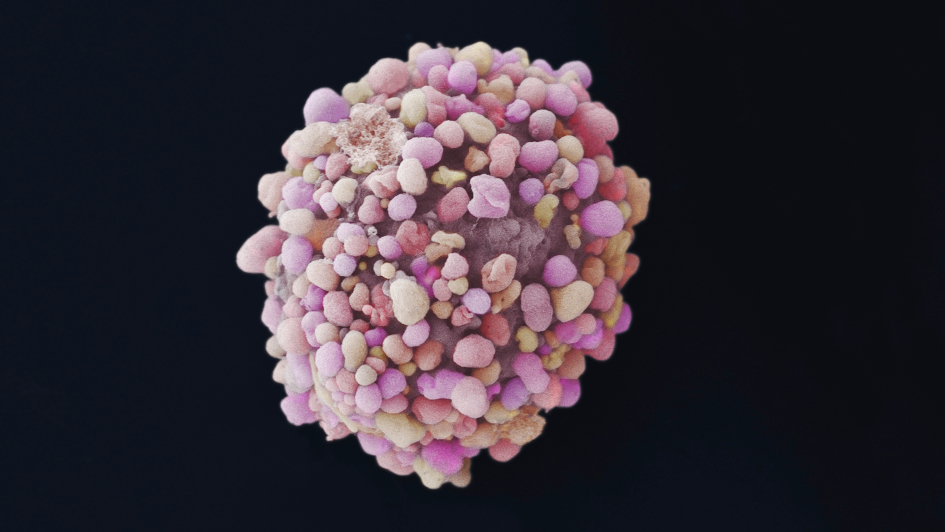
Image: Breast cancer cell. Image credit: Anne Weston, Francis Crick Institute via the Wellcome Collection. Licence: CC BY-NC.
ICR scientists discovered a new ‘evolution-busting’ drug that blocks one of cancer’s key escape routes from chemotherapy. The drug is now being evaluated in a clinical trial and could ultimately be used to treat aggressive breast cancers.
In a study published in the Journal of Medicinal Chemistry, the research team described the painstaking creation and testing of a new drug called BOS172722, an inhibitor of the protein kinase MPS1.
A key challenge our medicinal chemists faced was that their most promising MPS1 inhibitors were broken down too quickly by liver enzymes, and hence not capable of reaching tumours in patients. The team overcame this challenge by adding a stabilising ‘methyl group’ – a small chemical group consisting of one carbon and three hydrogen atoms – to the molecular core, greatly enhancing the stability of the compound.
The study was co-led by Dr Swen Hoelder and former ICR Head of Chemistry Professor Julian Blagg, building on previous work at the ICR led by Professor Spiros Linardopoulos.
Dr Hoelder said:
“It was a painstaking but rewarding endeavour to build our new drug, following research at the ICR to discover and validate MPS1 as a cancer target. It’s a pleasure to have discovered the drug and see it enter a clinical trial building on our work and it is great example of the interdisciplinary team work required to bring new therapies to patients.”
The study was supported by funding from partners including Cancer Research UK, the Cancer Research Technology (CRT) Pioneer Fund, Sixth Element Capital and Breast Cancer Now.
December 2019
New role identified for key protein which could be a potential target for tumours with BRCA mutations
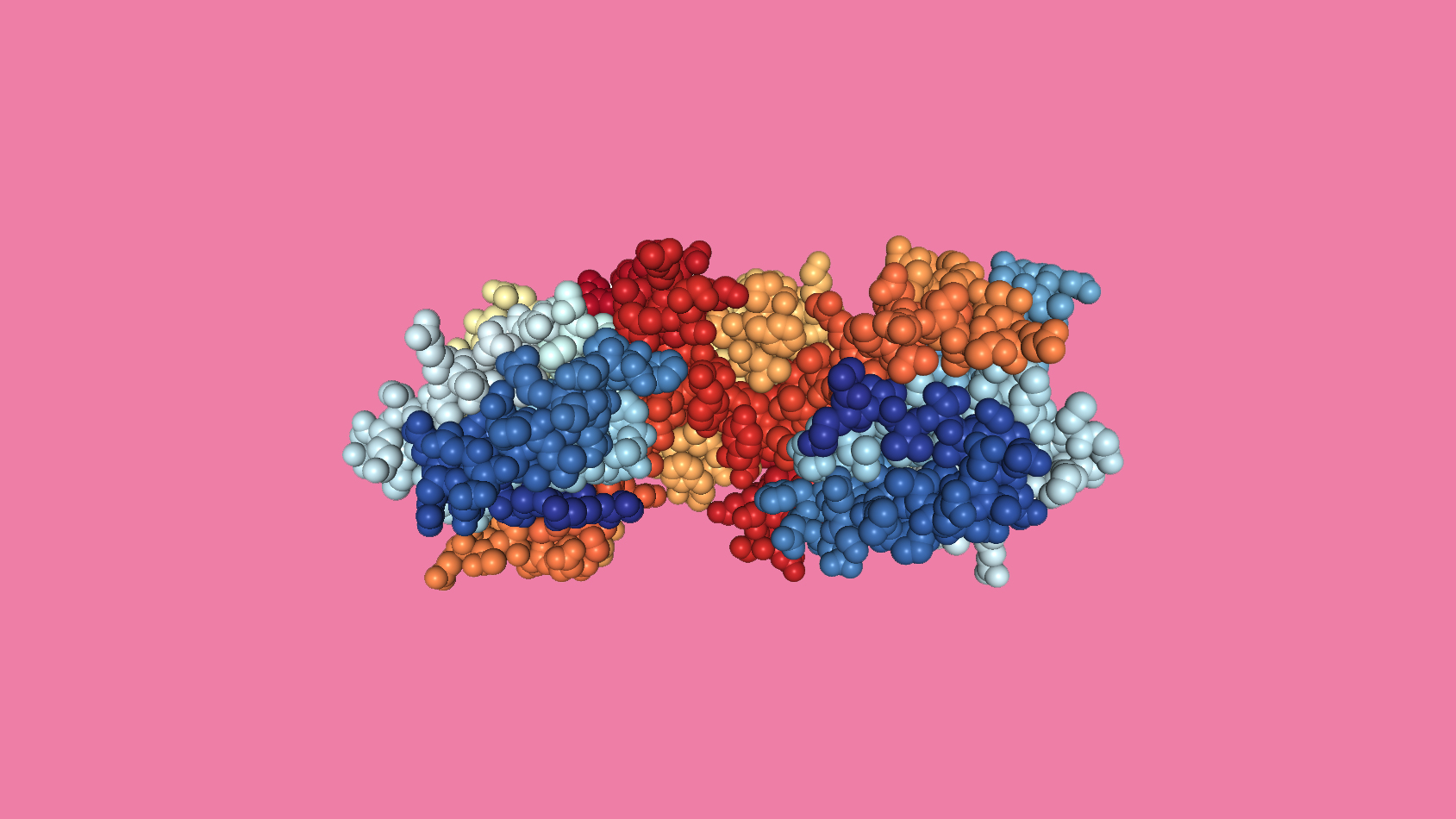
Image: Crystal structure of EXD2 exonuclease domain in spacefill format. Image generated from PDB
ICR scientists uncovered a previously unknown role for the enzyme EXD2 – identifying it as a potential drug target for cancer therapy.
The researchers found that EXD2 is essential for ensuring that the process of DNA replication can take place without introducing genetic errors. In cells with faults in the BRCA genes, which are involved in repairing DNA, the additional loss of EXD2 causes a build-up of genetic errors and eventually cell death – a process known as ‘synthetic lethality’.
The research suggests that blocking the function of EXD2 could be an effective treatment strategy for cancers with mutations in the BRCA genes. The ICR has separately pioneered use of synthetic lethality as a way of treating BRCA-mutant cancers through a class of drugs called PARP inhibitors.
Study leader Professor Wojciech Niedzwiedz, Professor of Molecular Cancer Biology said:
“Our research shows that tumours with mutations in the BRCA genes rely on EXD2 to stay alive. This is really exciting from a clinical perspective, as it means EXD2 is a potential target for developing new precision-guided anti-cancer drugs.”
The research, published in Molecular Cell, was funded by the ICR and Cancer Research UK.