Light Microscopy Facility
In our world-class microscopy suites, researchers have access to a fleet of temperature and CO2-controlled microscopes capable of performing high-resolution confocal and super-resolution microscopy, single-molecule and multi-position time-lapse experiments.
The ICR was also the first research organisation in the UK to acquire a lattice light sheet microscope. Using ultra-thin lattices of Bessel beams, the instrument enables very rapid imaging of cellular sub-sections without the effects of photo-toxicity.
“By working with industry to acquire the lattice light sheet scope, we can now analyse highly dynamic processes such as cell division with unprecedented resolution in both time and space.” — Professor Jon Pines, Head of Cancer Biology
For more information, contact:
- Kai Betteridge, Light Microscopy Facility Manager (Chelsea)
- Louise Howell, Light Microscopy Facility Manager (Sutton).
Light Microscopy Facility Chelsea
The Light Microscopy Facility in Chelsea is managed by Kai Betteridge and supported by Queenie Lai and Ross Scrimgeour.
It consists of 13 state-of-the-art microscopes for live and fixed cell analysis. The facility is composed of three parts:
1. Advanced light microscopy facility: two widefield microscopes for widefield imaging of samples and for long time lapse experiments of live cells, three confocal microscopes for different confocal imaging and for time-lapse experiments of live cells, three light sheet microscopes, one TIRF microscope for Total Internal Reflection of Fluorescence Microscopy.
2. High content screening facility: four plate based high-throughput microscopes.
3. State-of-the-art image analysis equipment and software: commercial software and dedicated image analysis computers (Celigo, MetaXpress, Aivia, Imaris, SlideBook, Harmony, Signals Image Artist, Velocity), open-source software (FIJI ImageJ, Cell Profiler and Cell Profiler Analyst, Python/Napari, Ilastik), other resources (including custom scripting eg MATLAB/Python, high-performance computing, ICR research data storage).
Light Microscopy Facility Sutton
The Light Microscopy Facility in Sutton is managed by Louise Howell. It consists of six advanced light microscopes geared towards state-of-the-art automated slide scanning and live cell confocal and fluorescence imaging.
The equipment includes two confocal microscopes for imaging fixed and live samples, and four slide scanners for brightfield and fluorescent slides.
There are also multiple dedicated image analysis computers with commercial software (eg Zeiss Axio Scan, Zeiss Zen software, Zeiss Zen Intellesis, Metacyte, In Situ Imaging System software, Tissue FISH module, V-slide software and VSViewer, Definiens Tissue Studio software, Hamamatsu NZ Acquire and NDP view 2, Duolink Image Tool), and open-source software (FIJI ImageJ, QuPath, Cell Profiler and Cell Profiler Analyst).
The Mariana 100 widefield is a Zeiss Axio Observer Z1 MarianasTM Microscope attached with a Cool LED pE 300 fluorescence excitation source. The system is equipped LED excitation light sources at wavelengths best suited for live cell imaging. Bright field imaging is set up on this system and it is capable of Differential Interference Contrast (DIC) imaging. The system is suitable for looking at standard histology specimens, or any experiment that may need to detect colour using transmitted light.

The Basic Spinning Disk is a Zeiss Axio Observer Z1 MarianasTM Microscope attached with a CSU-X1 spinning disk unit built by Intelligent Imaging Innovations (3i). The system is equipped with 6 solid state lasers at wavelengths best suited for live cell imaging and FRET .

The Advanced Spinning Disk is a Zeiss Axio Observer Z1 MarianasTM Microscope attached with a CSU-W spinning disk unit built by Intelligent Imaging Innovations (3i). The system is equipped with 6 solid state lasers at different wavelengths for a range of imaging modalities. The system is also equipped with a 355nm UV laser which can be controlled simultaneously with the spinning disk (using an mSwitcher). This feature is most commonly used by our users to locally cause DNA damage and observe the effects of this damage in real time.

The Leica SP8 is a point scanning confocal microscope ideal for imaging fixed cells and tissue mounted to a slide. It is equipped with 4 gas lasers with different wavelengths for a range of imaging modalities. The microscope has a fully adjustable pinhole and uses a combination of Photomultiplier tubes (PMT’s) and Hybrid Detectors (HyD) for detecting image.
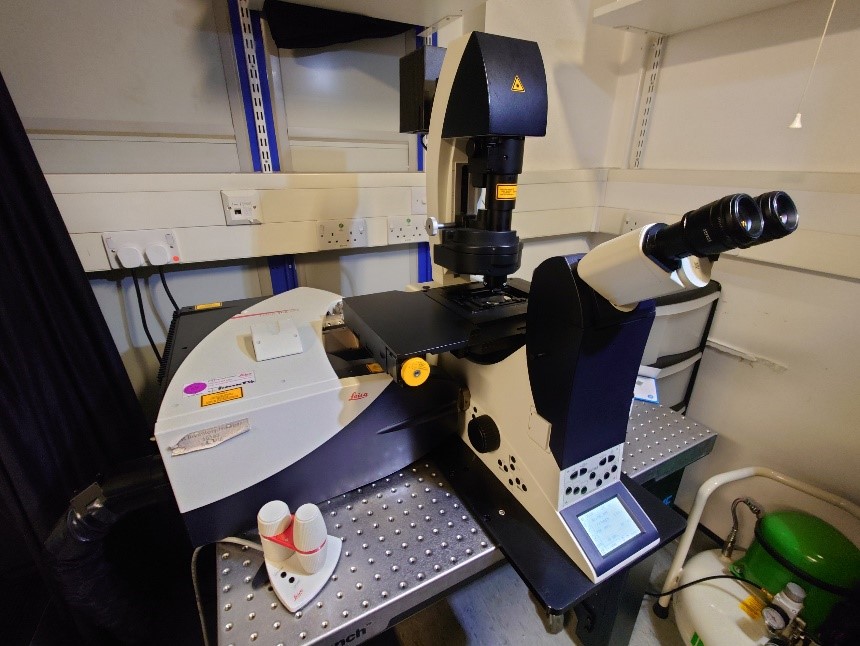
The lattice light sheet utilises extremely thin lattices (0.5 μm) made from Bessel beams to illuminate the sample. The thin sheet makes it possible to sub-section through a cell but still collecting all the light. The main benefits are extremely low photo toxicity and extremely fast imaging. The system does support one super resolution method, structured illumination microscopy (SIM).
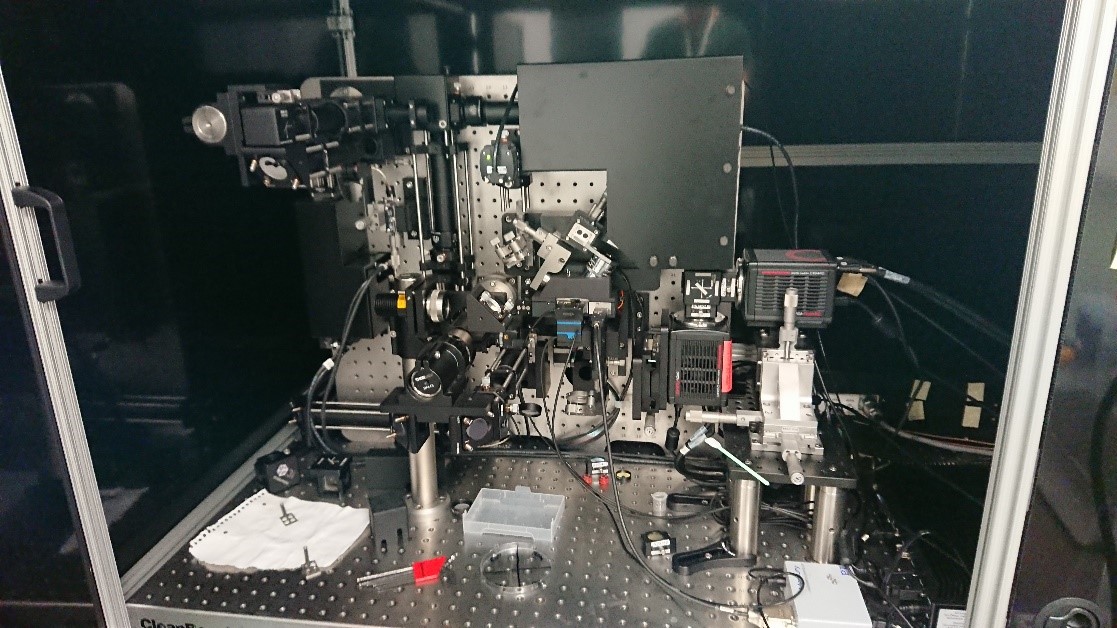
The MLS is a Dual-View inverted Single Plane Illumination Microscope (DiSPIM) that uses a Gaussian light sheet. Because it is possible to image from two sides this microscope can provide images with iso-tropic resolution. It is very useful for larger structures such as organoids as well as for single cells.
-v3.jpeg?sfvrsn=74d7fb75_3)
The CTLS is a dual-side illumination lightsheet microscope designed for imaging whole cleared organs or tissues. It incorporates a macro zoom microscope with high NA objectives and a Spatial Light Modulator (SLM) to optimise lightsheet for high resolution imaging.
-v2.jpeg?sfvrsn=ca15801a_3)
The TIRF is a Zeiss Axio Observer Z1 Marianas TM Microscope attached with a 3i Vector TIRF for Total Internal Reflection of Fluorescence Microscopy. The microscope is also capable of super resolution imaging (STORM and PALM).
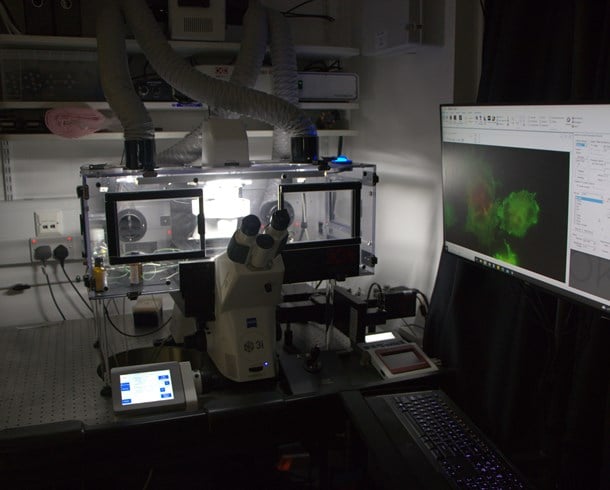
Widefield system that can scan plates very fast. This instrument also comes with an automated plate loading robot and is great for performing very large screens quickly.

High content spinning disc confocal microscope. This instrument comes with an automated plate loading robot as well as a liquid handling system. It is ideal for performing very large high content screens with unrivalled resolution and speed.

ImageXpress is a high content spinning disc confocal microscope.

Homebuilt microscope for automated lightsheet imaging of multiwell plates. This instrument has been built as part of a collaboration between Christopher Dunsby’s Lab (at Imperial College London) and Chris Bakal’s Lab (ICR).

The equipment is built on a fully motorised Axio Observer 7 inverted microscope stand with environmental chamber for control for temperature and C02 and uses Zen blue 3 acquisition and analysis software. It has a fast multiplexed Airyscan 2 detector which allows super-resolution imaging at faster speed. It is suitable for imaging fixed cells/tissues and live cell imaging/time courses, photo-manipulation techniques such as FRAP, imaging in multiple positions and stitching of large areas.

The Zeiss LSM700 is a point scanning confocal microscope on a motorised inverted Zeiss Axio Observer Z1, with stage top incubation for temperature and CO2 control of samples so both fixed and live samples can be imaged. The system is equipped with 4 solid state lasers at different wavelengths.
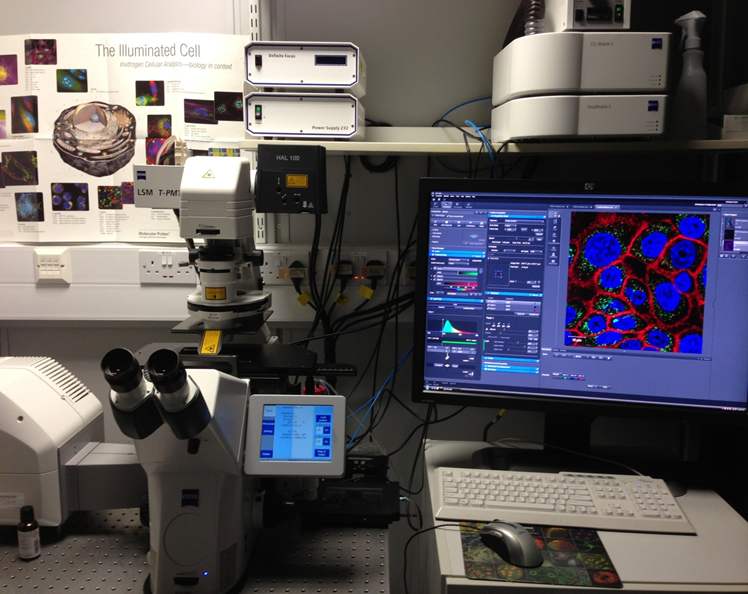
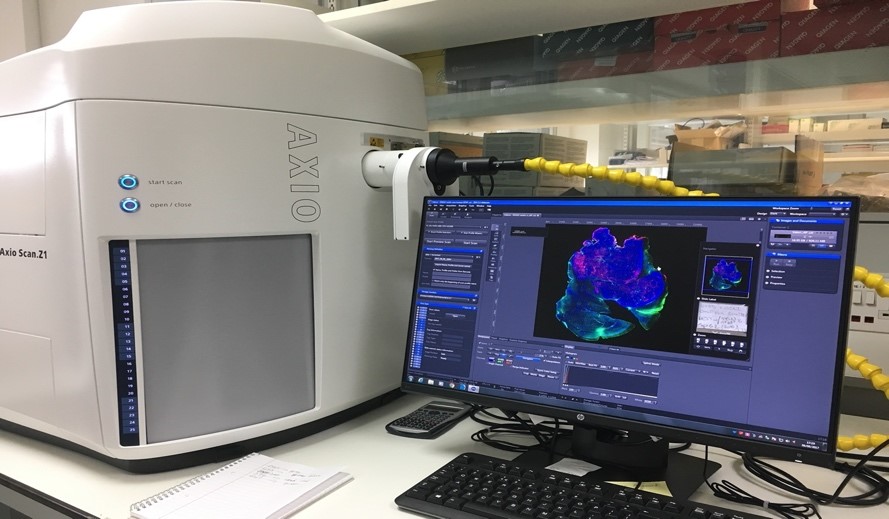
The Zeiss Axio Scan Z1 is a high end brightfield and fluorescent slide scanner with a 100 slide capacity. It has one preview specimen/label camera and two imaging cameras. High end fluorescence illumination with HXP and fast separated filter wheels. Zeiss Plan-Apochromat objectives lenses and a Zeiss Fluar 5x for overview and coarse focus. Zeiss Zen2 Slide scan imaging and analysis software.
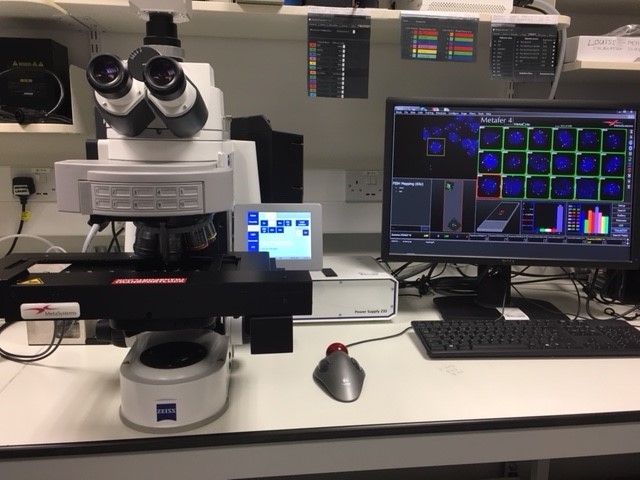
Automated system for the unattended detection and analysis of signals in interphase cells and tissue sections based on the scanning platform Metafer 4. With Zeiss Axio Imager Z2, fully motorised microscope with 10 position filter turret, 8 FISH filter sets, various objective lenses, with auto oiling pump and nozzle. Metacyte software finds and identifies nuclei or cells based on morphologic criteria, automatically stores position data, acquires fluorescence signals within objects from different focal planes and in up to 6 colour channels.
The Hamamatsu Nanozoomer is a high throughout automated slide scanner for brightfield and fluorescent slides with a 320 slide capacity. Fast scanning speeds (approx. 35s at 40x mode: area 15mm x 15mm) make this system ideal for quickly acquiring your H&E and IHC images.

.png?sfvrsn=364266fe_1)
