Animal research
The Institute of Cancer Research believes that animal research is essential to understand how cancers develop and behave within a whole organism, and how to treat it effectively.
We use animal studies alongside many other non-animal experimental approaches and they are crucial in building up a complete picture of cancer biology. Our research using animals has helped drive advances in cancer treatment that are benefiting people with cancer all over the world today.
Find out how our research using animals has helped drive advances in cancer treatment that are benefiting people with cancer all over the world today.
Under UK law animals can only be used for research if there is no appropriate alternative. All our research proposals are thoroughly assessed before approval to ensure that there is no alternative to the use of animals, and that the studies will provide valuable information that will ultimately help cancer patients.
The ICR is strongly committed to the highest standards of animal welfare in all research studies, and has led the development of best practice in this area. We also support the principles of the 3Rs – replacement, refinement and reduction of use of animals for research – and are working to further develop alternative experimental techniques.
We are signatories to the Concordat on Openness on Animal Research, and are helping to drive best practice in how we communicate about our research using animals with the public and in our scientific publications.
I’m proud of the work we do at the ICR to understand cancer and to help deliver innovative new treatments for people suffering from it. I’m also proud of the responsible way we do our animal research and how we share our experiences with other researchers and the public. Animal research plays an important role in our scientific discoveries and in providing benefits for cancer patients around the world.
Professor Kristian Helin, Chief Executive of the ICR

Using cancer epidemiology to understand the causes of the disease

#BreakTheBias: Scientists showcase their lives outside the lab
Statistics
Animals are used in our research to help us understand the mechanisms that underpin cancer, such as the growth and spread of tumours, and to develop new ways of diagnosing, treating and preventing the disease.
Studies will provide valuable information that will ultimately help cancer patients.
-held-in-a-cardboard-tube-tmb-0small-(1).jpg?sfvrsn=8dd89661_1)
Each regulated procedure is assigned a severity which describes the animals experience.
The statistics for animal use are based on animals used in regulated procedures.
The definition of a regulated procedure from the legislation is any "procedure applied to a protected animal for a qualifying purpose which may have the effect of causing the animal a level of pain, suffering, distress or lasting harm equivalent to, or higher than, that caused by the introduction of a needle in accordance with good veterinary practice."
For each procedure a 'severity' is assigned which describes the experience of the animal as categorised below:
- Sub-threshold. These procedures may result in below threshold severity. For example, the procedure was expected to cause pain or suffering equal to, or higher than, that caused by inserting a hypodermic needle but the actual experience was in fact less than this. Examples of this include:
- The breeding of genetically altered animals under project licence authority but without a harmful phenotype
- Dispensing medication in food where the animals ate normally and suffered no ill-effects
- Mild. These procedures may cause short-term mild pain, suffering or distress and/or minor changes in well-being or condition. Mild procedures can include:
- Anaesthesia
- Non-invasive imaging such as a MRI scan
- Short term social isolation
- Taking a blood sample
- Moderate. These procedures may cause short-term moderate pain, long-lasting mild pain, suffering or distress. They can result in a moderate impairment to well-being. Examples include:
- Surgery under general anaesthetic
- A modified diet
- Exposing the animal to something from which they would normally flee
- Severe. These procedures are where it is likely that the animal will experience severe pain, long-lasting moderate pain, suffering or distress and severe impairments to its well-being or general condition. Examples include:
- Any test where fatalities are expected
- Testing a device that could cause pain or death were it to fail
- Breeding animals with genetic disorders that are expected to result in severe and persistent impairment of general condition
- Non-recovery. Where the animal is placed under general anaesthetic before the start of a procedure and is humanely killed without regaining consciousness.
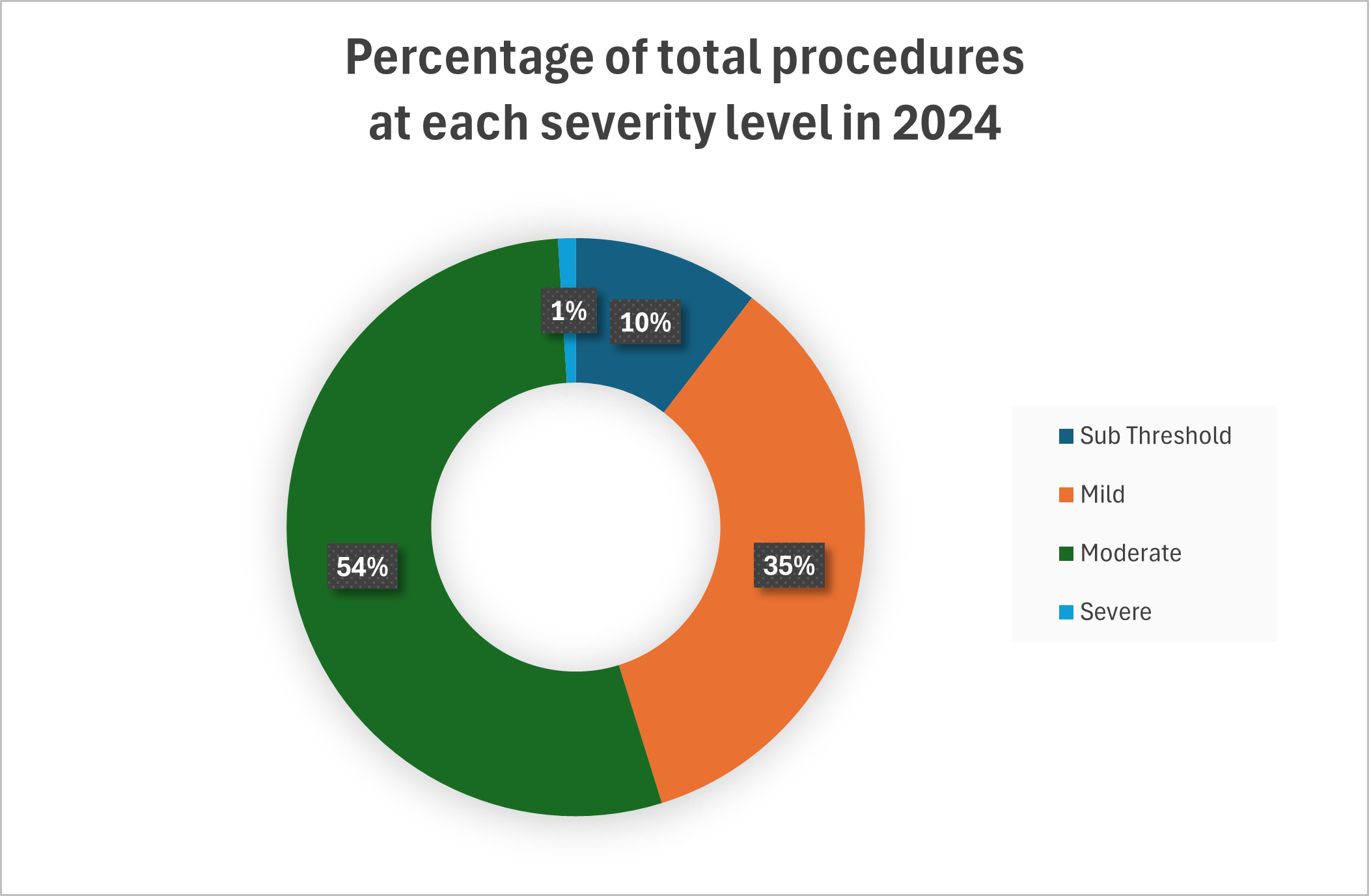
The actual severities for the years 2021 to 2023 are detailed in the table below.
Severity | Procedures in 2021* | % of total in 2021 | Procedures in 2022 | % of total in 2022 | Procedures in 2023 | % of total in 2023 | Procedures in 2024 | % of total in 2024 |
Sub Threshold | 4174 | 23.0 | 6197 | 27.3 | 3483 | 14.3 | 2413 | 10.4 |
Mild | 4705 | 25.9 | 5602 | 24.7 | 9849 | 40.4 | 8048 | 34.6 |
Moderate | 8977 | 49.5 | 10399 | 45.9 | 10716 | 44.0 | 12459 | 53.6 |
Severe | 274 | 1.5 | 432 | 1.9 | 220 | 0.9 | 229 | 1.0 |
Non recovery | 18 | 0.1 | 29 | 0.1 | 105 | 0.4 | 100 | 0.4 |
Total | 18148 |
| 22659 |
| 24373 |
| 23249 |
|
*covid
Animals are used in our research to help us understand the mechanisms that underpin cancer, such as the growth and spread of tumours, and to develop new ways of diagnosing, treating and preventing the disease.
Our work at the ICR mainly uses mice, which can grow tumours which mimic those of human cancer patients. Studies of cancer in mice mimic the complex way tumours grow and spread in people with cancer.
Mice can be easily genetically altered to allow us to study the genetic causes of cancer and reproduce tumour types which naturally occur in humans in the correct tissues and body systems, for example we use mice which have been genetically engineered so they develop the equivalent of children’s cancers affecting the brain and nervous system. We have had great success in developing treatments for these cancer types which have helped many children.
In other studies we implant cancer cells from a patient’s tumour into a mouse organ. This has the advantage that we can study a human cancer with a whole organism.
We also conduct some studies in rats. We use rats when we need to work on a slightly larger animal, if for example the research involves studying blood vessels, involves surgery or some types of imaging.
Each year we record the number of animals that we have used at the ICR, to report back to the Home Office. Information from the last 14 years is shown in the table below.
Year | Mice | Rats |
2010 | 50,483 | 346 |
2011 | 46,625 | 188 |
2012 | 27,781 | 299 |
2013 | 39,949 | 275 |
2014 | 29,194 | 103 |
2015 | 27,849 | 269 |
2016 | 21,314 | 94 |
2017 | 26,320 | 645 |
2018 | 23,523 | 291 |
2019 | 25,771 | 51 |
2020 | 17,713 | 73 |
2021 | 19,998 | 186 |
2022 | 22,991 | 98 |
2023 | 24,358 | 15 |
Our projects
Before the use of any regulated animal species in research, the research proposal and justification needs to be submitted as a project licence, which is reviewed by our Animal Welfare and Ethical Review Body (AWERB). If approved, the proposal is then submitted to the Home Office for further review and for the licence to be granted to permit the research to proceed.
All project licences contain a Non-Technical Summary detailing the research objectives, requirement for the use of animals, justification of species, animal numbers and severity of procedures, how harms are minimised and how the principles of Reduction, Refinement and Replacement (the 3Rs) have been and will be applied. The active project licenses being undertaken at the Institute currently are listed below.
We have two state-of-the-art facilities containing a maximum capacity of approximately 6000 rodent cages. These facilities ensure we carry out good-quality research in controlled conditions. Our animals live in an ultra-clean environment in cages that protect them from pathogens. Our facilities provide filtered air, ultraclean water, and sterile bedding and nesting, carefully monitored to maintain a high-quality environment. We enrich cages with items that our animals can interact and play with, as an important part of looking after their welfare. Our Operational team ensure that all equipment and cages are maintained in good condition as well as managing our cage processing facility which includes modern washing and automated cage processing equipment. On average cages are changed every 7-10 days, washed and sterilised before reuse.
Common procedures include giving drugs through injections or through a tube into their stomach. We also do surgical procedures on some mice and rats under anaesthetic – for example to implant tumour cells under the skin.
We will try new treatments in mice before selecting the most promising to take forward into clinical trials in patients. In studies of new cancer treatments, researchers test whether a potential drug can shrink a tumour or slow its growth. To obtain robust and meaningful data, it is important that scientists can accurately measure the size of tumours in mice to see whether the treatment being tested is having any effect.
We often use imaging techniques like MRI, commonly also used for human patients, to measure the size of tumours in mice. The mice will be scanned in small versions of the same machines used for patients in the hospital. Using imaging techniques such as these means that we can reduce the numbers of animals we use in our studies as we monitor the progress of the cancer in a single mouse rather than multiple mice.
Animal welfare
Animal welfare is very important to us at the ICR and we ensure that our animals are well cared for. We strongly support the principles of the 3Rs – replacement, refinement and reduction of use of animals in research – which have been widely adopted across research organisations.

The research community is always looking for ways to improve welfare and minimise the use of animals in research. The Institute of Cancer Research strongly supports the principles of the 3Rs – replacement, refinement and reduction of use of animals in research – which have been widely adopted across research organisations.
Replacement - means using alternative experimental techniques that don’t involve animals.
Refinement - involves looking at the way that experiments are carried out, and the ways animals are housed and cared for, to reduce any pain or distress and improve welfare.
Reduction - involves using fewer animals or getting more information from the same number of animals.
Wherever we can we replace the use of animals in research, refine experimental procedures and minimise the number of animals used in experiments. We work closely with organisations such as the National Centre for 3Rs (NC3Rs) and the RSPCA to work on new approaches and technologies to minimise the use of animals and improve animal welfare.
All establishments which use animals in research must have processes for reviewing the ethics of proposed projects and the adoption of the 3Rs. At the ICR this is done by our Animal Welfare and Ethical Review Body, which includes members of the public.
Animal welfare is very important to us at the ICR and we ensure that our animals are well cared for. The Biological Services Unit have around 12 animal technologists and care staff responsible for carrying out health monitoring checks and husbandry every single day. To ensure the highest standards of animal care and service to the researchers using the facility, we provide staff with a continuous programme of internal and external training, including formal qualifications, in order to support career progression in animal technology and facility management.
We have led the development of best practice in animal welfare in cancer research, helping develop the Guidelines for the welfare and use of animals in cancer research which are used by cancer researchers in the UK and worldwide. You can read more about the legislative landscape, ethics and welfare.
All research projects at the ICR using animals are reviewed by our Animal Welfare and Ethical Review Body, which includes members of the public. The group examines the ethics of all research projects proposed and ensures that all projects consider the 3Rs principles. It must agree that our work is necessary and will be sufficiently beneficial before we can do any research using animals.
The research community is always looking for ways to improve welfare and minimise the use of animals in research and has embraced the principles of the ‘3Rs’ – replacement, refinement and reduction of use of animals in research. You can read more about our work on the 3Rs.
We have led the development of best practice in animal welfare in cancer research. Our former Chief Executive, Professor Paul Workman chairs a committee sponsored by the National Cancer Research Institute which developed Guidelines for the welfare and use of animals in cancer research which are used by cancer researchers in the UK and worldwide.
We have Named Animal Care and Welfare Officers who oversee the care and welfare of the animals at the ICR. The Officer advises our researchers on welfare issues and ensures we do everything we can to minimise suffering and care for the animals we use in the best way we can. We also have a veterinary surgeon who can advise on the health and welfare of animals.
Animal research in the UK is governed by strict laws. The Animals (Scientific Procedures) Act 1986 sets out specific controls over the way research is done.
In the UK research can only be carried out when authorised by the Home Office. For every research project using animals, there must be a licence for the research project itself, for the individual researchers carrying out the project and for the institution where the project is taking place.
Licences are only granted to projects when the potential results can justify the use of animals, where the research can’t be done without the use of animals and when discomfort to the animals is kept to a minimum. The establishment must have appropriate veterinary facilities and look after animals properly. All work must be carried out by highly trained and qualified staff who hold a personal licence and are competent to carry out the procedures, and whose competency is routinely monitored.
Concordat on Openness on Animal Research
In 2014, The Institute of Cancer Research signed the Concordat on Openness on Animal Research, developed with organisations across the biomedical research sector, and committed to be more open about the use of animals in research.
The Concordat sets out how signatory organisations will ensure members of the public have access to accurate information about how animals are used in their research and the role of animal research generally in science and medicine.
In signing the Concordat, we agree to fulfil the Concordat’s four commitments and will report annually on our progress against each of them:
· We will be clear about when, how and why we use animals in research
· We will enhance our communications with the media and the public about our research using animals
· We will be proactive in providing opportunities for the public to find out about research using animals
· We will report on progress annually and share our experiences
The ICR played an active role in developing the Concordat and strongly welcomes its aims to improve communication about animal research.
You can read more about our commitment to openness in this blog post from Professor Paul Workman, Professor of Pharmacology and Therapeutics at the ICR, outlining why we are committed to greater transparency about use of animals in research, and explaining why the responsible use of animals is essential.
We have gone beyond the requirements of the Concordat in helping drive best practice in open communication about our research using animals.
In 2014, an episode of Panorama focusing entirely on the pioneering work of the ICR and The Royal Marsden aired on BBC One. The Panorama team was given unprecedented access to ongoing research at the ICR and The Royal Marsden, including our studies using animals. The documentary showed how we are trialling potential new ALK-targeting drugs in mice before aiming to take the most promising candidates to children in clinical trials.
It also followed our work using mouse avatars where a sample of a patient’s cancer is implanted into a mouse to seed the formation of a tumour, allowing researchers to test for mutations that may make the patient’s cancer susceptible to specific drug treatments.
By signing the Concordat on Openness on Animal Research, we have reiterated our commitment to being transparent about when, how and why we use animals in our work.
Professor Kristian Helin, Chief Executive of the ICR
-without-tumour7594d5f2-1cdb-4498-9b61-b6a7c639f67e.jpg?sfvrsn=a33603c6_3)