Epigenetics and Genome Stability Group
Professor Jessica Downs’s group investigates how DNA packaging into chromatin influences the stability of genetic information, and studies how chromatin and its regulation can influence the development and progression of cancer.
Research, projects and publications in this group
By investigating how chromatin, or epigenetic regulation, impacts on genome stability, we hope to identify novel therapeutic targets and biomarkers for the treatment of cancer.
Professor Jessica Downs
Deputy Head of Division:
Epigenetics and Genome Stability
Professor Jessica Downs is Deputy Head of the Division of Cancer Biology. She is investigating the interplay between epigenetics and genome stability. The goal of this research is to understand how the packaging and organisation of DNA in cells helps to maintain its integrity and prevent tumourigenesis.
Researchers in this group
 .
.
Alison obtained her BSc in Biomedical Science at the University of Sheffield. She then completed her MSc degree in Molecular Medicine and her PhD in the Department of Surgery and Cancer at the Imperial College London. In April 2019, she joined Professor Down’s team at the ICR and her research is focusing on chromatin modifiers in cancer, working jointly with Professor Raj Chopra.
 .
.
Karen completed her undergraduate BSc degree in Pharmacology and an MSc in Biomedical Science at the National University of Ireland, Galway. She graduated from the Centre for Chromosome Biology in Galway with a PhD in Biochemistry in 2017. Karen started in Professor Downs' lab in 2018, where she is investigating synthetic lethal drug candidates in cancers which have mutations in the SWI/SNF chromatin remodelling complexes.
 .
.
Email: [email protected]
Location: Chelsea
Susana completed her BSc degree in Applied Biology at the University of Minho, and her MSc degree in Oncology at the University of Porto, both in Portugal. She then obtained her PhD in Cancer Sciences at The University of Manchester. In January 2019, she joined Professor Downs’ lab at the ICR and is investigating the contribution of chromatin dynamics to DNA damage responses and genome stability.
Professor Jessica Downs's group have written 55 publications
Most recent new publication 2/2025
See all their publicationsThe DNA in our cells is packaged by association with proteins, including histones, to form chromatin. The structure of chromatin is regulated by modifications and remodelling enzymes to dynamically allow access to the DNA during cellular activities such as replication, transcription, and repair. Alterations in chromatin structure and composition can have profound and heritable, or epigenetic, effects.
We are investigating how chromatin, or epigenetic regulation, impacts on genome stability. Areas that we have focused on include understanding how chromatin structure and its regulation directly influences DNA repair, chromosome segregation, and DNA replication.
By understanding more about these pathways, we can not only generate mechanistic insights into cellular functions, but also identify novel therapeutic targets and biomarkers for the treatment of cancer.
For more information, please visit our external lab website: thedownslab.org
A major focus of the lab is the SWI/SNF family of chromatin remodelling complexes. This family is made up of a number of multisubunit complexes that have been shown to be important for maintaining genome stability (for review, see Harrod, Lane and Downs, DNA Repair 2020). Work from our lab and others identified multiple different mechanisms by which SWI/SNF remodellers do this, such as DNA double strand break repair, sister chromatid cohesion, and DNA damage checkpoints.
Notably, genes encoding SWI/SNF subunits are also mutated in human cancers with striking frequency. For example, approximately 40% of clear cell renal cell cancers have deleterious mutations in the PBRM1 subunit of SWI/SNF. We performed a meta-analysis of SWI/SNF misregulation in cancer (in Harrod, Lane, and Downs, DNA Repair 2020) and found that around 20% of cancers have inactivating mutations in SWI/SNF encoding genes. When amplifications are also considered, almost 30% of cancers – across a wide range of cancer types – have misregulation of at least one SWI/SNF subunit.
Our research is aimed at exploring two major areas. First, we want to gain a deeper understanding of the functions of SWI/SNF complexes and of what happens when subunits are missing or misregulated. These studies are particularly focused on SWI/SNF activities that impact on genome stability, but we also look at other functions that can shed light on how SWI/SNF biology changes in cancer cells. Second, we want to identify vulnerabilities of cells with SWI/SNF misregulation that we can use to develop new therapeutic strategies for patients with SWI/SNF deficient cancers.
For more information, please visit our external lab website: thedownslab.org
Recent discoveries from this group
-being-attacked-by-two-cytotoxic-t-cells-(red)-547x410.tmb-hbmobile.png?Culture=en&sfvrsn=8f59440b_2)
Deliberately damaging DNA could boost the effectiveness of immunotherapy in kidney cancer
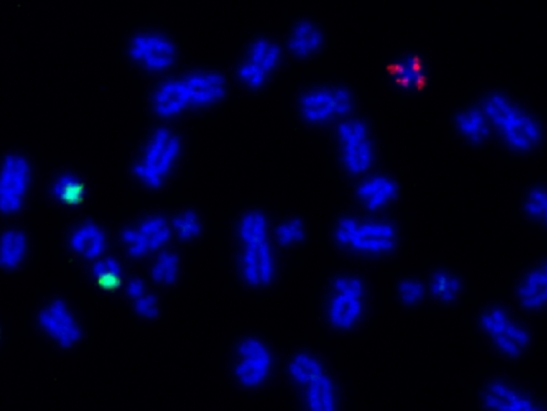
Common genetic mutation allows cancer cells to gain extra chromosomes which help them survive

ICR Discovery Club explores how exploiting cancer’s DNA weaknesses can unlock new treatment

 .
.