Cell Death and Immunity Group
Professor Meier and his group are studying the complex relationship between cell death, inflammation and adaptive immunity, and how this knowledge might be applied to deliver better anti-cancer drugs to the clinic.
Research, projects and publications in this group
We are using patient-derived organoids and animal models to investigate the role of cell death, immunity and inflammation in adaptation to tissue stress, treatment resistance and tumour surveillance.
This group shares more research detail through their X account.
Professor Pascal Meier
Group Leader:
Cell Death and Immunity
Professor Pascal Meier and his group are investigating the complex relationship between cell death, immunity and tumorigenesis. Particularly, they are focussing on the role of cell death and inflammation in adaptation to tissue stress, treatment resistance and tumour surveillance.
Researchers in this group
 .
.
Dr Fernando completed her MSc at the University of Queensland in 2008, and obtained her PhD from Griffith University, Australia in 2014. She worked as a postdoctoral researcher at the QIMR Berghofer, Australia and continued her postdoctoral training at Karolinska Institutet, Sweden. In 2020, she joined Professor Meier’s group at ICR as a Higher Scientific Officer for in vivo work.
 .
.
Email: [email protected]
Dr Sidonie Wicky John obtained her Diploma (Swiss equivalent to MSc) in Biology at the University of Neuchâtel, Switzerland. After a postdoc in Doug Kellogg’s lab at the University of California Santa Cruz, she joined the team of Professor Meier in the Breast Cancer Now Research Centre in 2009 as a Scientific Officer.
 .
.
Samuel Jouny is a Higher Scientific Officer in the Cell Death and Immunity Group. He specialises in the intraductal xenograft model and models of lobular breast cancer.
 .
.
Phone: +44 20 7153 5319
Email: [email protected]
Also on: jonathan-mannion-441b43147 , https://twitter.com/jonathanmannio1
Location: SuttonChelsea
Jonathan Mannion currently holds a BSc in Biomedical Science and an MRes in Cancer Biology, from Northumbria and Newcastle Universities, respectively. Shortly after completing his MRes, Jonathan spent 18 months at the MRC Laboratory of Molecular biology in Cambridge, where he studied the innate and adaptive arms of the type 2 immune response. In 2020, Jonathan joined Professor Pascal Meier’s research team as a CRUK-funded PhD student.
 .
.
Dr Tenev graduated from the Department of Biochemistry, Biological Faculty at the University of Sofia. He obtained his PhD at the Friedrich-Schiller University, Jena, Germany. In 2001 he joined Professor Meier's lab as a Postdoctoral Fellow, and in 2005 he was promoted to Staff Scientist.
 .
.
Email: [email protected]
Rebecca Wilson holds a BSc (Hons) from the University of Aberdeen and joined the Institute of Cancer Research as a Scientific Officer in 1996, working in the lab of Professor Chris Marshall. In 2000 she moved to Professor Meier’s Cell Death and Differentiation team in the Breast Cancer Now research unit.
Professor Pascal Meier's group have written 80 publications
Most recent new publication 11/2024
See all their publications"Tumour Cell Death is not an endpoint, but the beginning of an immune response"
Our aim is to harness cell death and immunity to defeat cancer.
Cell death is integral to the immune response and acts as a beacon, a second messenger, that guides both immune system and tissue micro-environment to ensure tissue repair and homeostasis. Dying cancer cells are also crucial to anti-tumour immunity by stimulating the cross-priming of CD8+ T cells (Figure 1).
However, not all cell deaths are equal in stimulating an anti-tumour immunity. While some type of cell death forms are immunogenic, others are immunologically silent or even tolerogenic, shutting down T-cell responses. Therefore, understanding which type of cell death is immunogenic, and which therapies induce them, is of paramount importance for anti-cancer therapies to maximise CD8+ T cell activation.
In our laboratory, we study the effect of cell death pathways on anti-tumour immunity.
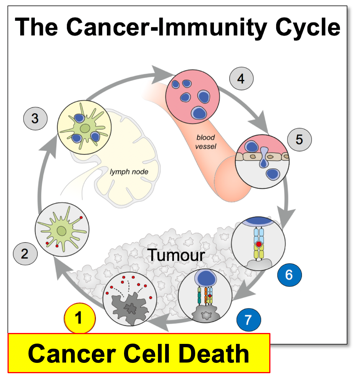
Figure 1. The cancer-immunity cycle (Chen et al., Immunity, 2013). Immunity in cancer is a cyclic self-propagating process. Each cycle starts with cell death and ends with a T cell response.
Our programme focuses on the crosstalk between cell death, inflammation and immunity in cancer. Immunogenic cell death is a successful dialog between a dying cell and a rightly disposed immune system.
If successful, this interaction promotes an immunogenic outcome proportional to the threat. Critical for the dialog are damage-associated molecular patterns (DAMPs), whose production varies depending on the type of cell death in quantity and quality.
How can we take advantage of the fundamental principles of immunogenicity (Figure 2)? Our improved understanding of the different types of cell death - apoptosis, necroptosis, pyroptosis, and ferroptosis - and their interconnectivity with innate and adaptive immunity has unearthed new opportunities to guide our immune system to fight cancer.
Our goal is to manipulate cell death signalling pathways so that they not only kill the cancer cell, but also guarantee an immunogenic outcome. This can be achieved using strategies that are based on ‘viral mimicry’. This simulates the presence of an imaginary pathogen, collaterally triggering an attack on the tumour and an immunisation against tumour antigens.
Additionally, since cancer treatment often fails because of the evolution of resistant clones, we aim to ‘steer’ cancer evolution by changing the fitness landscape of cancer cells while strengthening the one of surrounding healthy tissue.
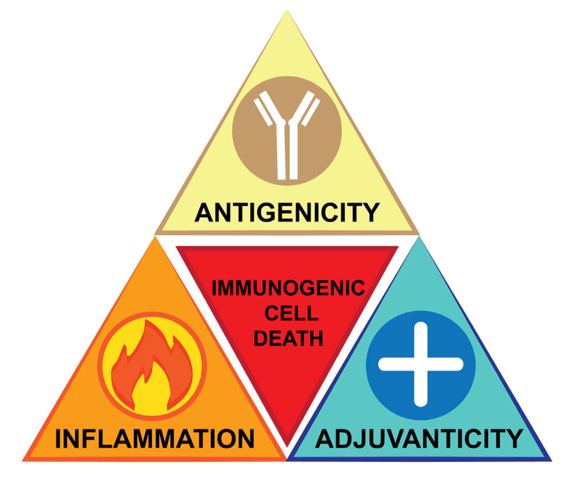
The overarching goal of our research programme is to identify and understand the molecular mechanisms behind immunogenic cell death so they can be manipulated in cancer therapies. Our research themes are (Figure 3):
Theme 1: Immunogenic cell death in cancer
• Identify regulatory mechanisms of necroptosis/apoptosis/pyroptosis and DAMPs production in the tumour
• Study the therapeutic consequences of immunogenic cell death on tumour/immune interactions in breast and lung cancer
Theme 2: Winners versus Losers: Cell Competition in tumour evolution, heterogeneity and drug resistance
• Develop novel anti-cancer treatment approaches that target the competitiveness features of the tumour microenvironment to convert super-competitor cancer cells into super-losers.
 figure 3: Research programme overview. Theme 1 (left): Immunogenic Cell Death (pushing current treatment regiments into immunogenic cell death). Theme 2 (right): Killing tumour cells by targeting the fitness landscape.
figure 3: Research programme overview. Theme 1 (left): Immunogenic Cell Death (pushing current treatment regiments into immunogenic cell death). Theme 2 (right): Killing tumour cells by targeting the fitness landscape.
- We established that IAPs (Inhibitors of APoptosis proteins) function at the crossroad of cell death regulation and inflammation; a view-point that is currently being exploited in clinical trials with pharmacological inhibitors of IAPs.
- We contributed to the characterisation of the ripoptosome, a large protein complex that can regulate cell death, mitosis, cell motility and cytokine production, with exciting implications for the design of novel therapeutic approaches aimed at selectively destroying apoptosis-resistant cancer cells.
- We reported the first pre-clinical application of an in vivo treatment protocol for soft-tissue sarcoma that directly engages RIPK1-mediated immunogenic cell death.
Professor Meier and his group are using patient-derived organoids and animal models to investigate the complex relationship between cell death, immunity and tumorigenesis. Since these pathways are conserved between different species, it will be important to understand evolutionary principles that cause human diseases. Particularly, the laboratory is investigating the role of cell death, immunity and inflammation in adaptation to tissue stress, treatment resistance and tumour surveillance.
 .
.

.png?sfvrsn=e5be7df6_2)