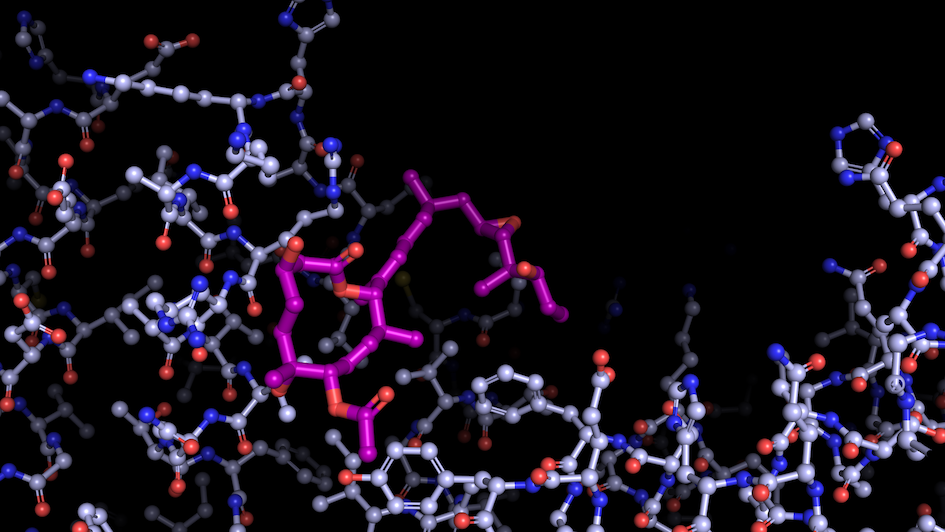
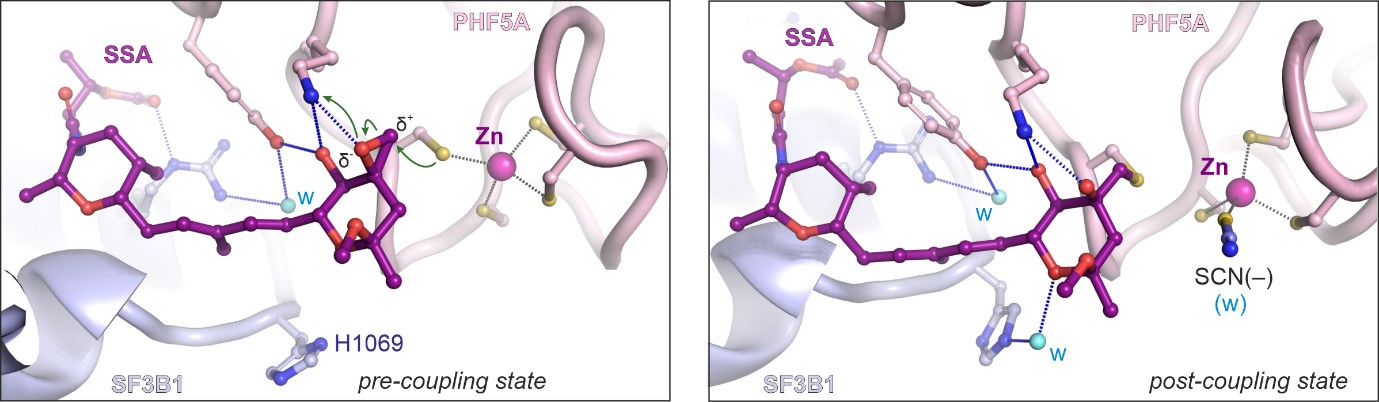
Image: “Like a constellation of atoms” – splicing modulator (purple) bound to a spliceosome subunit. Credit: Professor Vlad Pena.
Scientists have uncovered the inner workings of one of the most important and intricate ‘nanobots’ operating within our cells – using cutting-edge microscopy for visualising molecules almost at an atomic level.
Their new study published in Nature has unveiled the critical step that switches on the spliceosome – a piece of cellular machinery that enables cells to build complex proteins.
By uncovering in detail how the spliceosome is activated, scientists believe the discovery could pave the way to more effective design of cancer drugs which target it.
State of the art microscopy
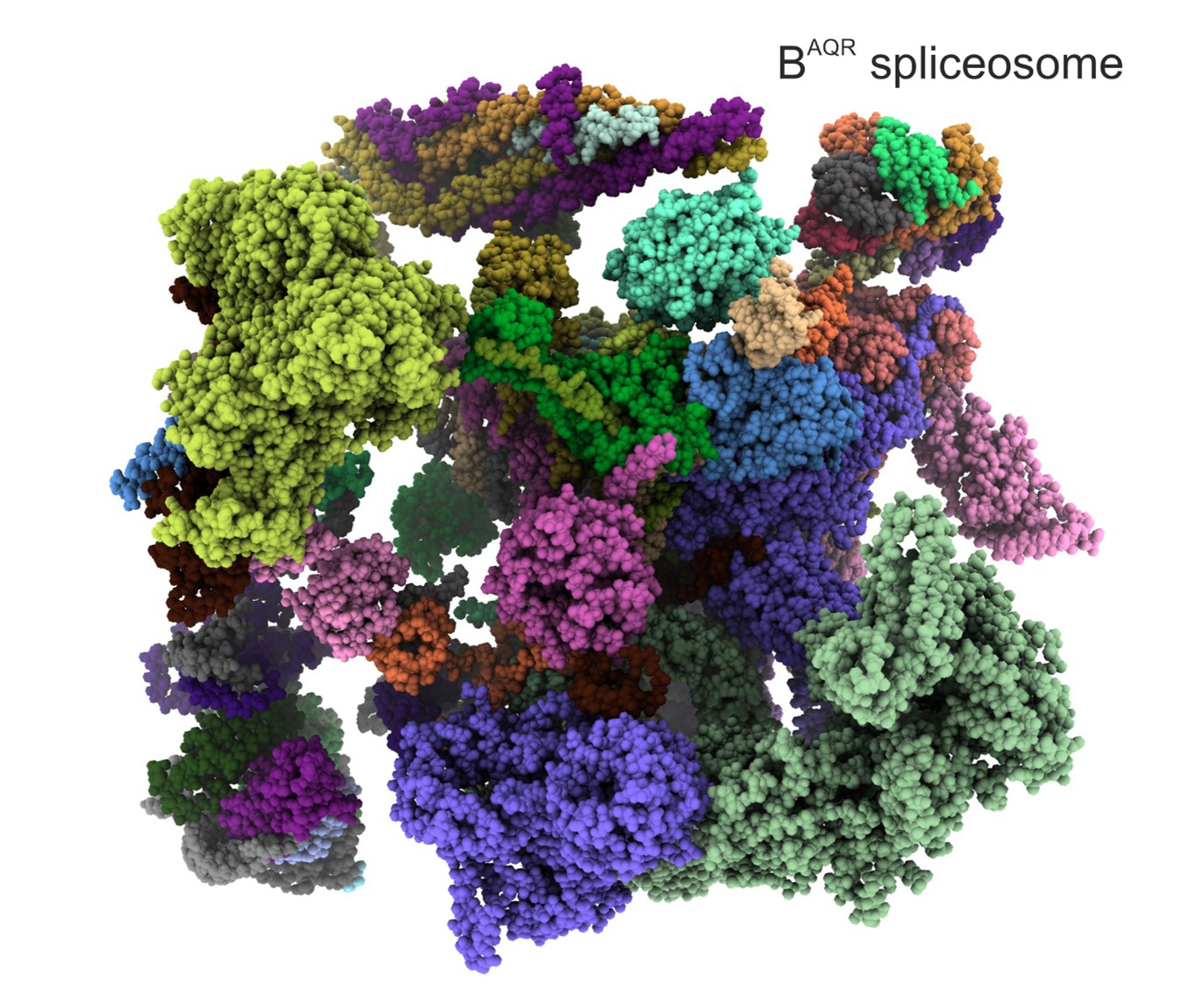
An international team of scientists from The Institute of Cancer Research, London, and the Max Planck Institute for Multidisciplinary Sciences in Germany employed state-of-the-art biochemical and cryo-electron microscopy (cryo-EM) methods to study the spliceosome in intricate detail and answer long standing questions about how it works.
The spliceosome operates like a nanobot, processing RNA – genetic instructions copied from DNA – in a key step to allow the building of complex proteins.
Powered by molecular motors called helicases, the spliceosome chops and changes RNA code to increase the complexity of the genetic instructions so that many different proteins can be made from a limited number of genes. This process is called splicing.
Splicing explains why humans, who only have around 20,000 genes, can produce hundreds of thousands of different proteins. It may also be a key reason for why humans can be so different from fruit flies, despite having a similar number of genes1.
Hallmark of cancer
Mutations in the spliceosome are a hallmark of cancer – they contribute to the production of abnormal proteins that fuel tumour growth or deactivate proteins that protect against cancer.
Scientists studied the spliceosome using cryo-EM – a cutting edge microscopy technique which involves rapidly freezing spliceosomes and bombarding them with electrons to obtain a 3D reconstruction of their molecular structure at almost atomic-level resolution.
They also employed advanced biochemical engineering methods to capture the spliceosome in the midst of activation – a feat never achieved before. This allowed them to dissect the precise molecular mechanisms occurring within the spliceosome, much like an engineer taking apart an engine but on a sub-microscopic scale.
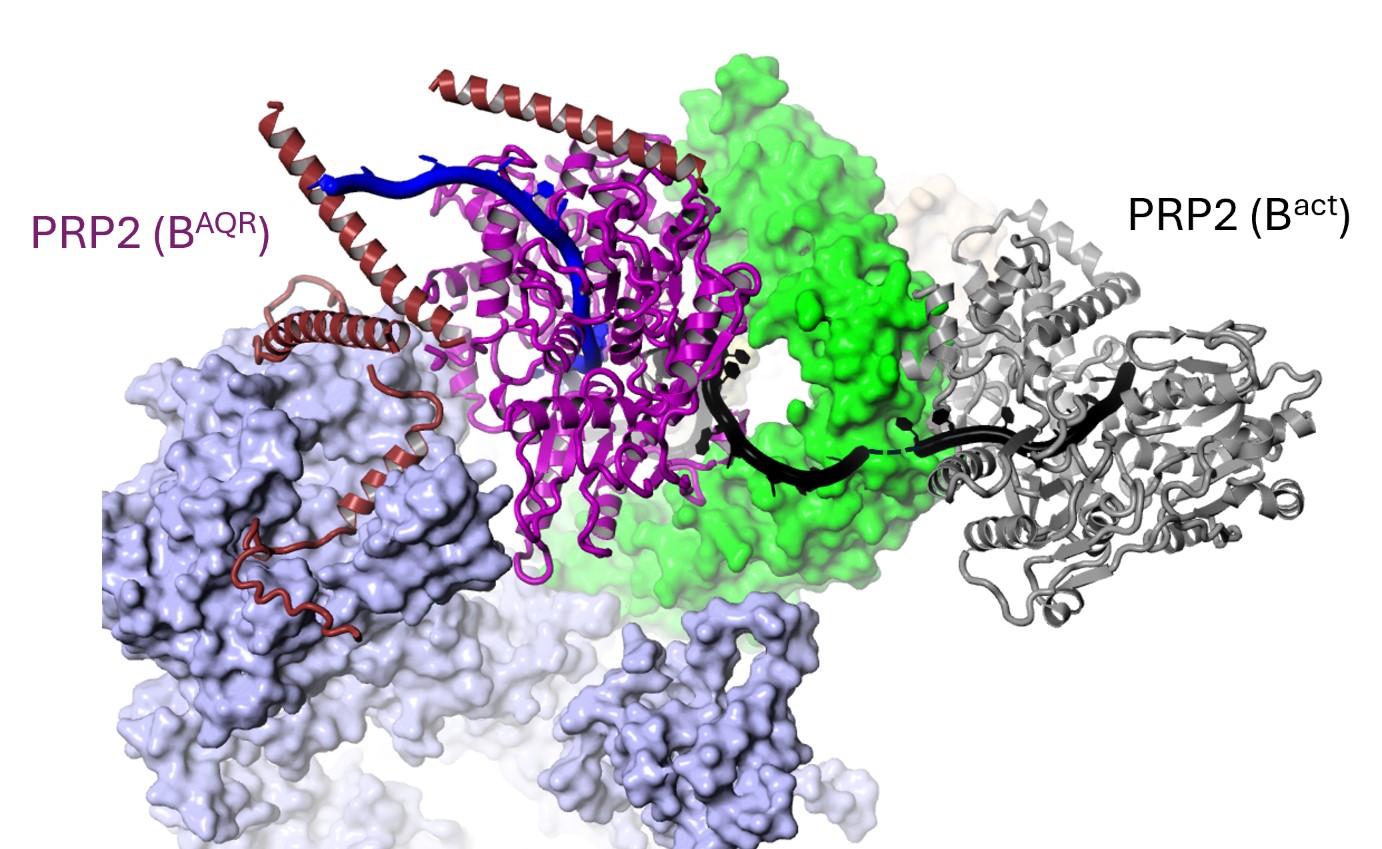
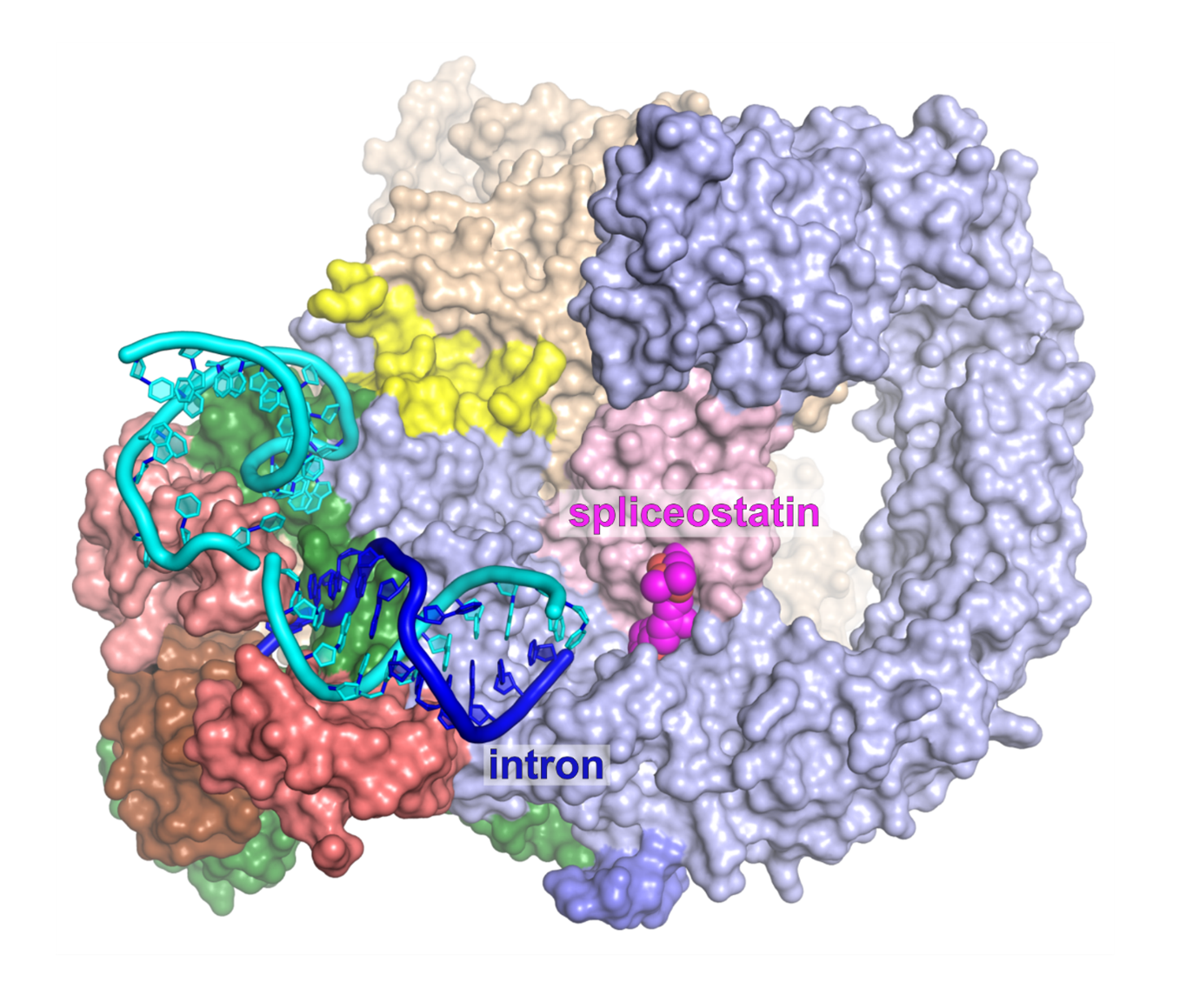
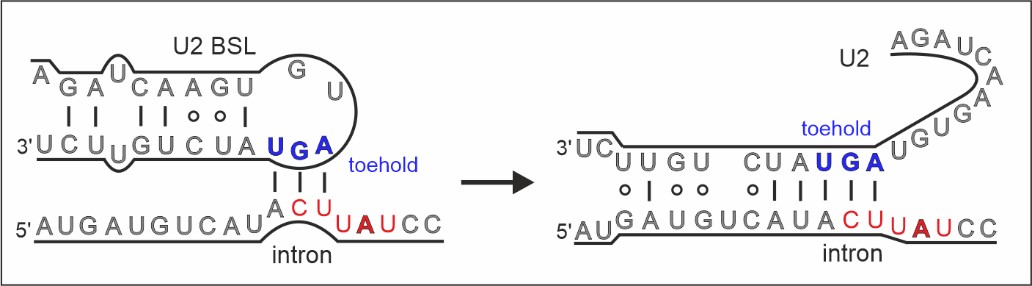
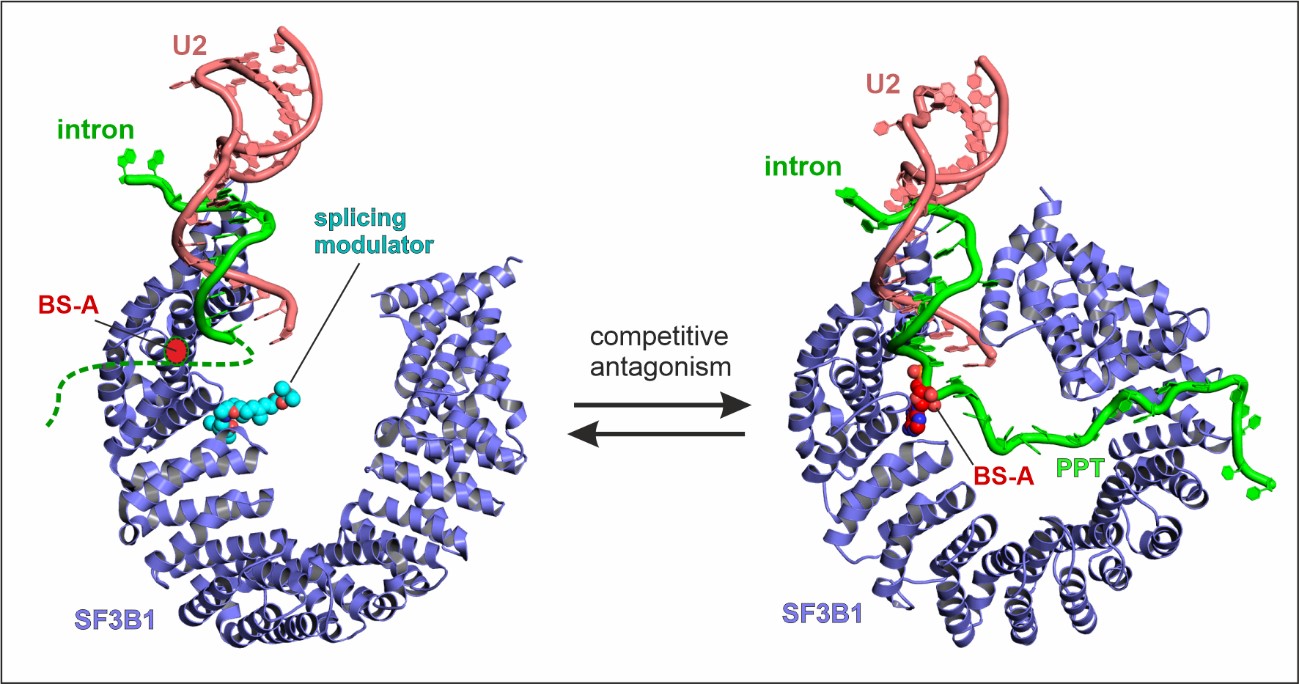
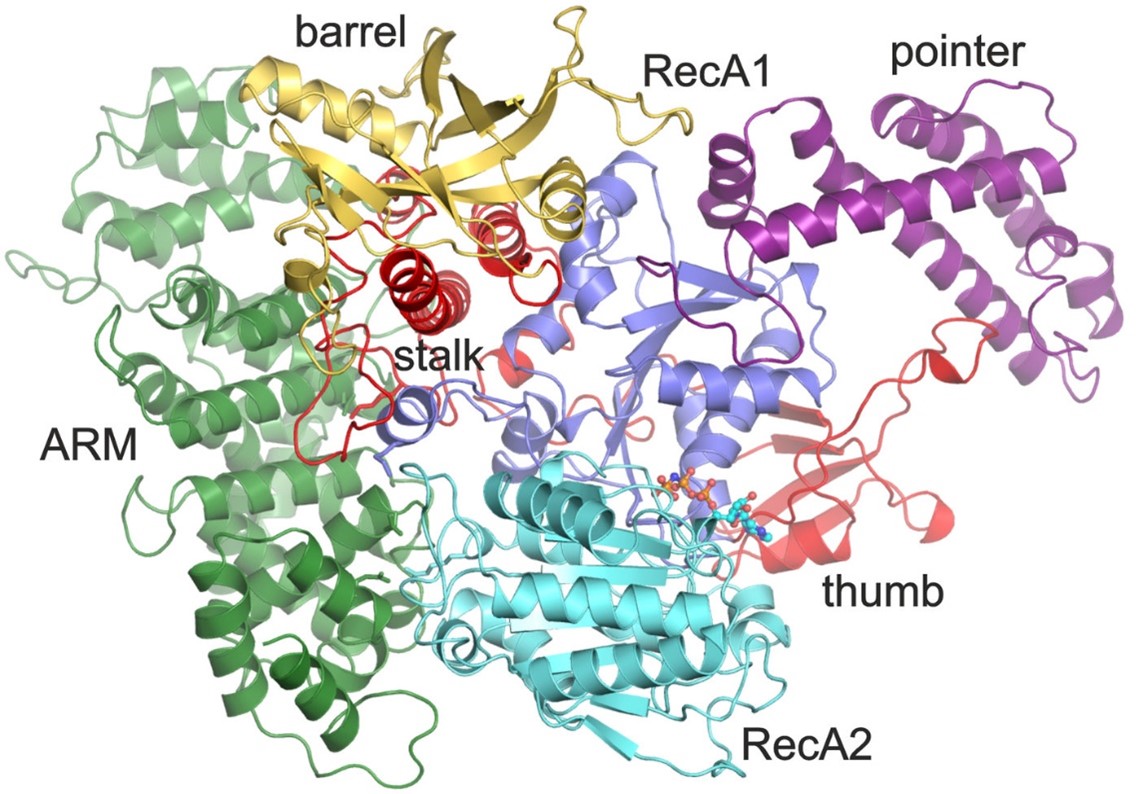
Of particular interest was a core spliceosome subunit called SF3B1 which is essential for spliceosome activation. SF3B1 is the most mutated spliceosome gene in cancer, in particular in leukaemia, uveal melanoma, pancreatic, and prostate cancer.
The researchers discovered that two molecular motors reshape SF3B1, and in doing so they kickstart splicing.
Firstly, they showed that a molecular motor called PRP2 interacts with SF3B1 and works in a completely new way than was previously thought for splicing helicases. Instead of staying on the outside of the spliceosome, PRP2 “walks” along the RNA strand being processed, all the way to the spliceosome’s core, rearranging the spliceosome structure as it travels and helping to switch the spliceosome into an active state. The researchers believe that other helicases may also work in this new and unexpected way.
Secondly, they found that a second motor named Aquarius also acts on SF3B1 and is essential to activate the spliceosome.
The findings represent a fundamental advance in our understanding of the spliceosome and how it is activated by helicases.
Professor Vlad Pena, Professor of Structural Biology and Gene Expression at The Institute of Cancer Research, London, who supervised the research team said:
“The spliceosome is an intricate nanobot that uses molecular motors to process genetic information. This information is passed on and forms instructions for building proteins.
"We used a new engineering technique to reveal that kickstarting the spliceosome requires the help of two distinct motors, PRP2 and Aquarius. This is a breakthrough finding in our understanding of how the spliceosome and its molecular motors operate.
“Splicing is often dysregulated in cancer, and we hope our work will inspire new research which will contribute to the design of new cancer drugs that can target the splicing process.”
Discovery could pave the way to better cancer drugs
Professor Kristian Helin, Chief Executive of The Institute of Cancer Research, London, said:
“These exciting findings represent a fundamental advance in our understanding of one of the most important and complex pieces of molecular machinery in our cells. The spliceosome not only enables complex life to exist but, when things go wrong, it can create proteins which help to fuel or sustain cancer.
“By illuminating the step-by-step process that activates the spliceosome, this research could pave the way to better cancer drugs to control the way it operates within cancer cells."
Help us revolutionise cancer treatment. We aim to discover a new generation of cancer treatments so smart and targeted, that more patients will defeat their cancer.

 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.