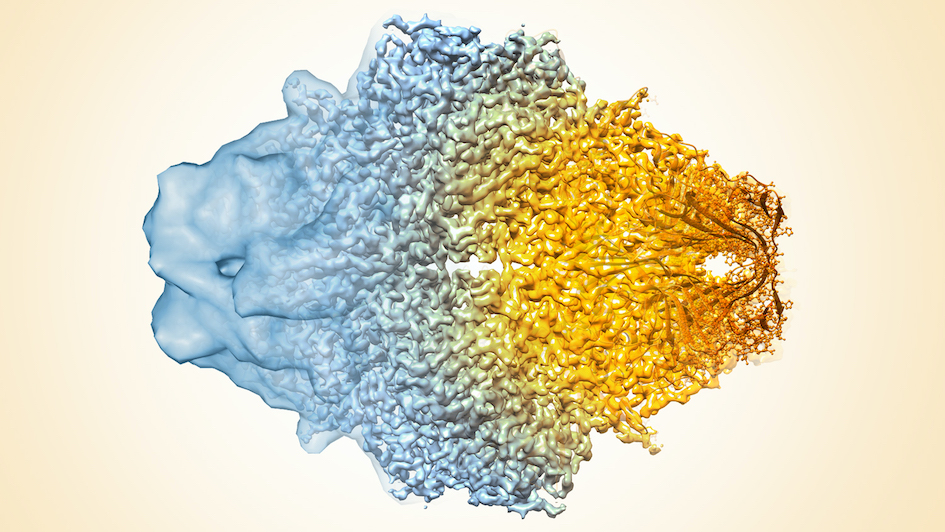
Image: Composite image of cryo-EM density maps of different resolutions. Credit: Veronica Falconieri and Sriram Subramaniam, National Cancer Institute (NIH) via Flickr, CC BY-NC 2.0
This is the second golden era of structural biology. And thanks to the London Consortium for Cryo-Electron Microscopy, researchers have a new way to make their contribution to an expanding field.
These were among the sentiments expressed by researchers to a packed auditorium at The Francis Crick Institute at the end of a busy day of talks from researchers of the London Consortium for Cryo-Electron Microscopy (LonCEM), which gathers together scientists from five of the leading scientific organisations in London, including the ICR.
The delegates were meeting to discuss their latest findings using cryo-electron microscopy, or cryo-EM, and our scientists showcased how we are using this exciting technology to drive forward advances in our understandings of cancer and how it could be treated.
Cryo-EM’s resolution revolution
Cryo-EM is a type of microscopy that exposes samples at extremely low temperatures to a beam of electrons to study the structure of molecules. It’s now a crucial tool for researchers in the discipline of structural biology to study the structures of proteins, nucleic acids and other biomolecules, the basis for discovering targeted drugs in cancer.
It isn’t a new technique, but cryo-EM is now at the forefront of structural biology research, having undergone something of a resolution revolution in recent years.
Advances in technology and software mean cryo-EM has come on in leaps and bounds in the past 10 years or so, allowing scientists to see proteins and other complex molecules at the atomic scale.
Samples are frozen at temperatures as low as -180°C, then placed in a beam of accelerated electrons. The information of how the specimen scatters the electron beam is then used to build a 3D map of the molecules.
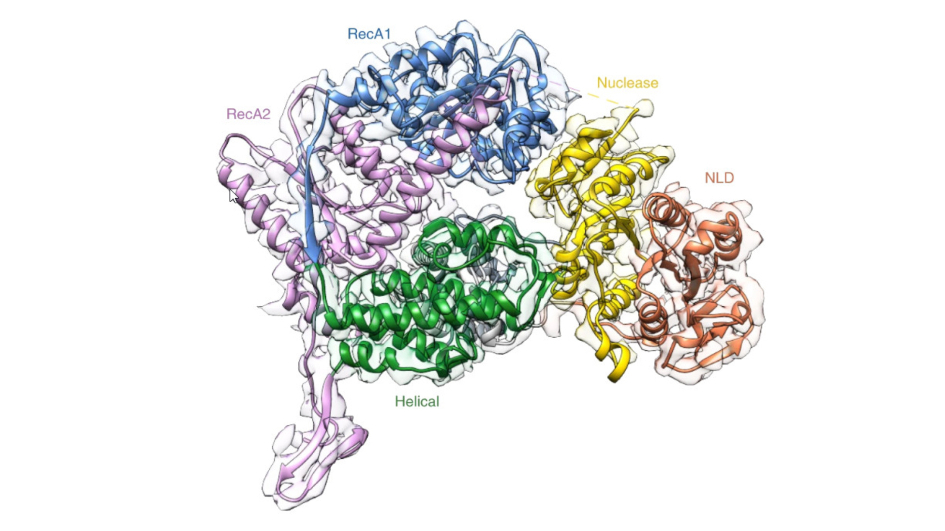
Cryo-EM can show the proteins that make up complex molecules, and how they are arranged at the atomic scale. This image shows the architecture of a molecule complex called XPF-ERCC1, from a research paper involving ICR scientist Dr Edward Morris.
Mapping biological processes is key to understanding and preventing disease, but for too long there have been blank spaces because the available technology hasn’t been able to image much of life’s molecular machinery. Now cryo-EM is changing all that.
Because it uses flash-frozen samples in solution, the molecules remain in a very similar state to how they would appear in their native environments. This means scientists can see molecules as they are found in our cells.
X-ray crystallography, another technique used to study the structure of biomolecules, needs crystallised samples that can be difficult or even impossible to produce for some types of molecule or large macromolecular machines.
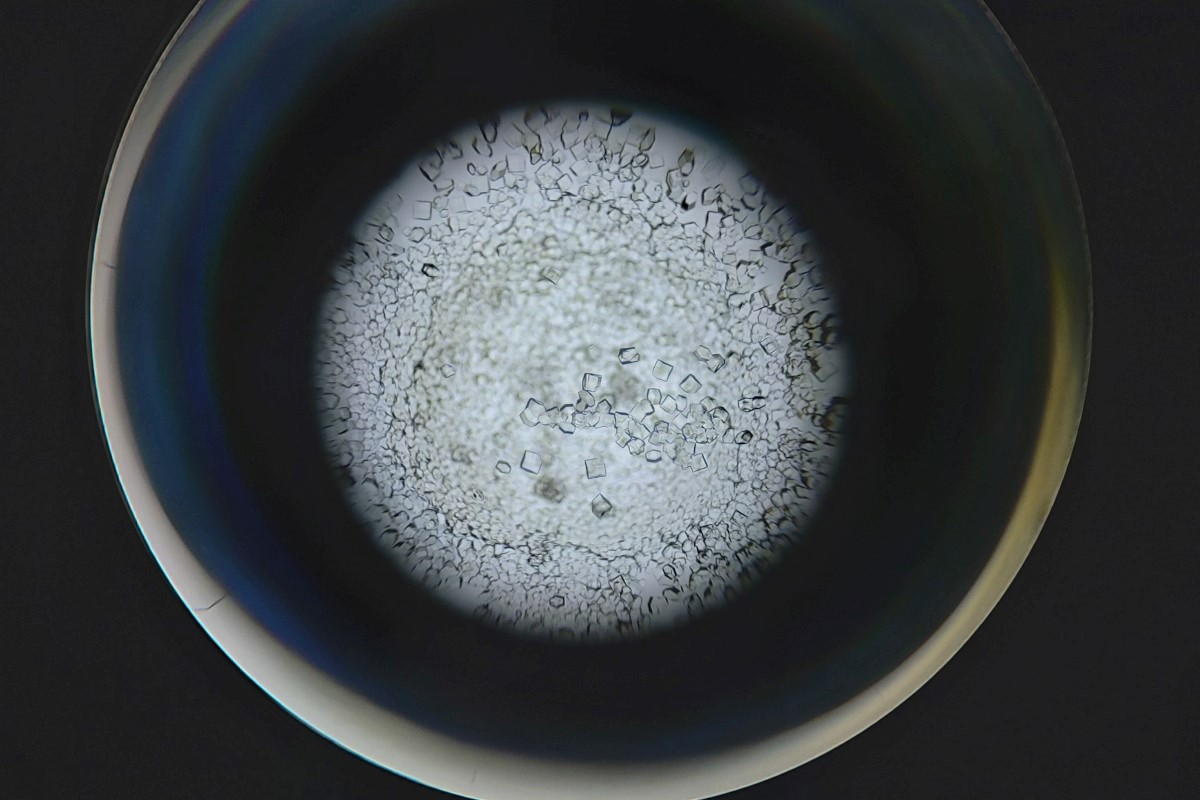
Protein crystals used for other imaging techniques. Not all proteins can be crystallised, making cryo-EM more flexible.
Cryo-EM has no such limitations, so it can look at many new molecules that couldn’t be imaged previously at this scale, opening up new avenues for discovery and innovation.
Researchers can freeze biomolecules mid-movement and visualise processes, which is important for both the basic understanding of life’s chemistry and for the development of new pharmaceuticals.
The London Cryo-EM Consortium
Scientists from the ICR, Imperial College London, King’s College London, Queen Mary University of London and The Francis Crick Institute have been working together through LonCEM, a joint initiative which is building a state-of-the-art cryo-EM facility at The Crick.
The consortium recently purchased a new microscope called the Titan Krios, thanks to a £3 million grant from The Wellcome Trust.
Scientists from each of the partner organisations described some of the exciting discoveries modern cryo-EM has helped to uncover, including the ICR’s Dr Sebastian Guettler, who spoke at the meeting.
Dr Guettler and his team are investigating an enzyme called tankyrase, which plays a key role in a type of cell signalling process dysregulated in 90 per cent of bowel cancer cases.
Many copies of the tankyrase protein assemble into long strings, and using cryo-EM they have obtained new clues into how these strings may control the activity of the enzyme.
The keynote lecture was given by former ICR scientist Professor David Barford, who is now a Programme Leader at the MRC Laboratory of Molecular Biology in Cambridge and Co-Head of the Division of Structural Studies.
Professor Barford was a key figure in setting up cryo-EM research at the ICR alongside ICR researcher Dr Edward Morris. Using cryo-EM, Professor Barford led a team that mapped the structure of one of the most complicated regulators of cell division, called the anaphase-promoting complex/cyclosome (APC/C).
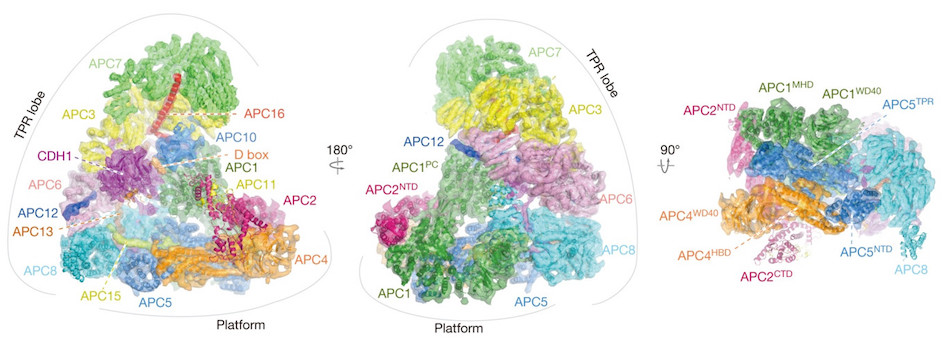
The Molecular architecture of the APC/C complex (reconstruction), captured in unprecedented detail using Cryo-EM.
The APC/C is an extremely large protein machine that plays a key role in regulating cell division, by controlling the chemical signals that tell a cell when it should divide.
Cancer hijacks the normal process of cell division, so detailed images of the gigantic protein complex are helping researchers like Professor Barford understand its role in this process, and could identify ways to target the molecule with drugs to treat cancer.
This was just one study led by ICR researchers that broke new ground in applying cryo-EM to cancer research. Other studies led by Dr Morris have uncovered other structures involved in cancer, including the Cullin-RING E3 Ligase 2 (CRL2), the COP9 signalosome, and the proteasome complex.
These sorts of findings show the potential of cryo-EM to understand how biological processes work, by revealing the intimate structures of proteins. As Sir Paul Nurse, Director of The Francis Crick Institute, said at the LonCEM meeting, cryo-EM is an amazing technology:
“It’s extraordinary to see how this technique has taken over structural biology. As a geneticist, I appreciate how cryo-EM helps to show the real nature of genes. It’s only really by understanding the structure of the gene products, the proteins, that we can actually put everything together.”
The future for cryo-EM
Thanks to the incredible detail of cryo-EM images and the versatility of the technique to study a wide range of molecules, it’s becoming the most popular tool for structural biologists to map the molecular machineries within our cells.
The LonCEM consortium will give ICR researchers the training and access to explore the structures of proteins and their complexes that play a role in cancer using cutting edge cryo-EM.
From these structures, scientists can better understand the processes that help cancer cells survive and grow, which could lead to new ways to treat the disease.