Genome Stability and Innate Immunity Group
Dr Christian Zierhut's group study how chromosomal aberrations that frequently occur in cancer promote intracellular immune responses, and how these control cell fate decisions.
Research, projects and publications in this group
Our group aims to generate an understanding of innate immune responses during cancer development and treatment in order to suggest new therapeutic strategies.
Dr Christian Zierhut
Group Leader:
Genome Stability and Innate Immunity
Dr Christian Zierhut leads the Genome Stability and Innate Immunity Group. His research focuses on how chromosomal aberrations that frequently occur in cancer promote intracellular immune responses, and how these control cell fate decisions.
Researchers in this group
Dr Christian Zierhut's group have written 14 publications
Most recent new publication 1/2024
See all their publications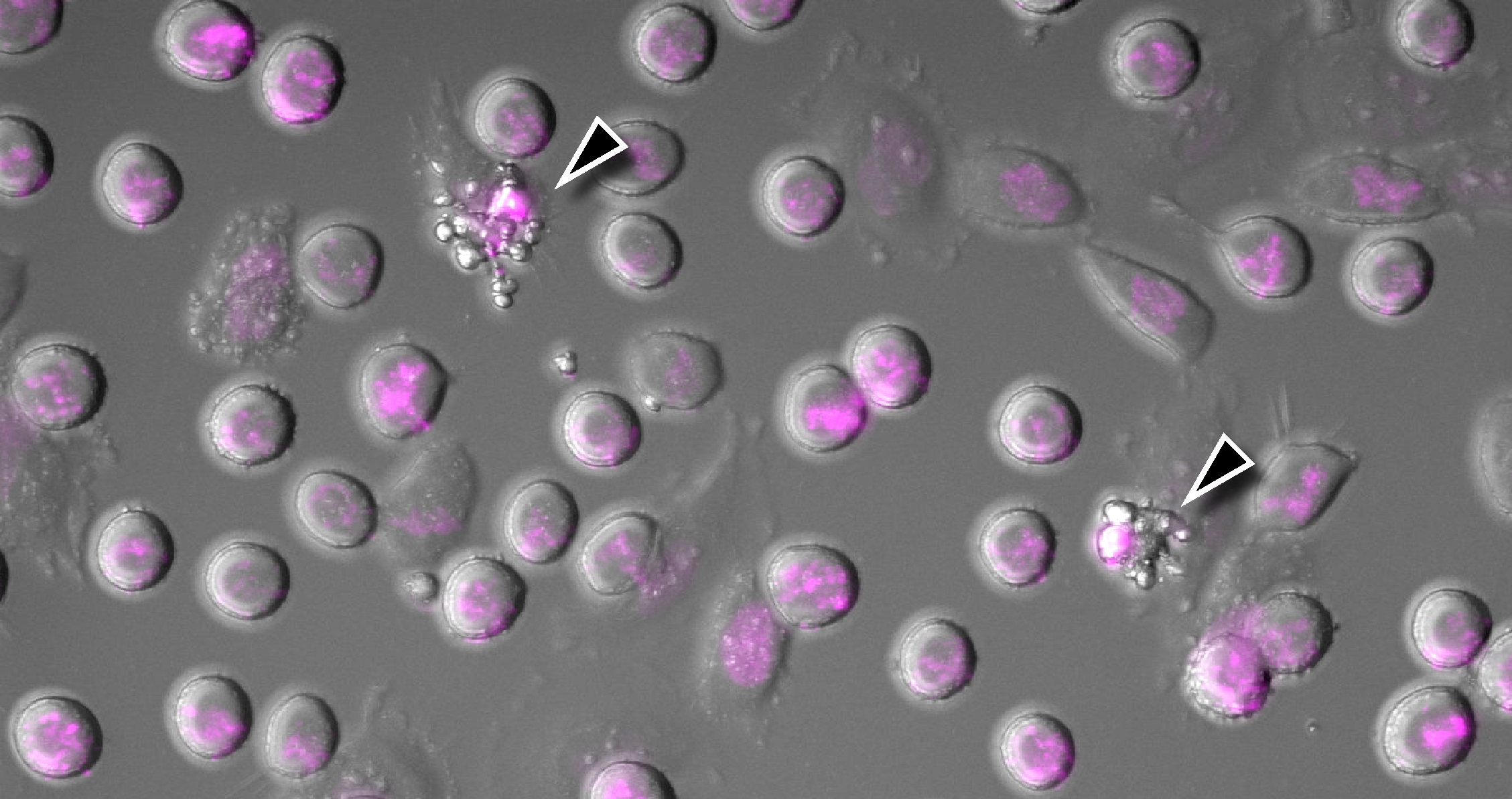
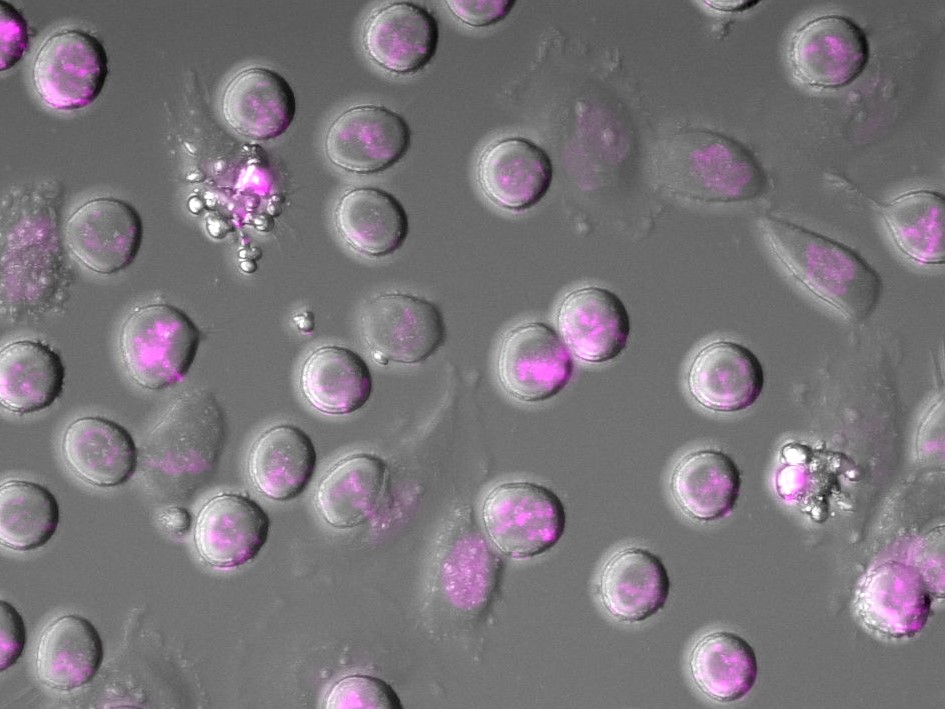
Image: Phase contrast image of HeLa cells expressing fluorescent histone H2B (magenta) arrested in mitosis using the chemotherapy drug Taxol. Arrowheads indicate cells undergoing cGAS-promoted apoptosis. Credit: Dr Christian Zierhut
There are two branches of the immune system - adaptive and innate immunity. While adaptive immunity responds to ever changing pathogen components through the generation of antibodies, antibodies to one pathogen type are unlikely to protect against another. In contrast, by responding to general pathogen components, innate immunity can provide protection against many pathogen types. One such general pathogen component is the genetic material, DNA, itself.
DNA of pathogens invading a cell is sensed by a number of innate immune sensors, foremost the cyclic GMP-AMP synthase cGAS. Intriguingly, despite the presence of large amounts of endogenous DNA within host cells, self-DNA generally does not activate cGAS. We previously provided evidence that this is because the chromatin organization of host DNA is incompatible with cGAS activation, thus silencing cGAS signalling during normal cell growth. In contrast, in response to genotoxic stress, cGAS can eventually be activated, regulating inflammatory responses, cellular senescence and cell death. Ultimately, activation of innate immunity via cGAS is critical for cancer development, chemotherapy and immune therapy.
We are currently investigating three major questions in order to understand the silencing of cGAS on self-DNA, and the relief of silencing upon genotoxic stress:
- How is cGAS inactivated on chromatin?
- How does genotoxic stress lead to cGAS activation, and how is this co-ordinated with other intracellular signalling?
- How does cGAS control cell fate after genotoxic stress?
By understanding these questions, we hope to provide explanations for the behaviour of cancer cells during cancer development and treatment, and to reveal new therapeutic avenues.
 .
.