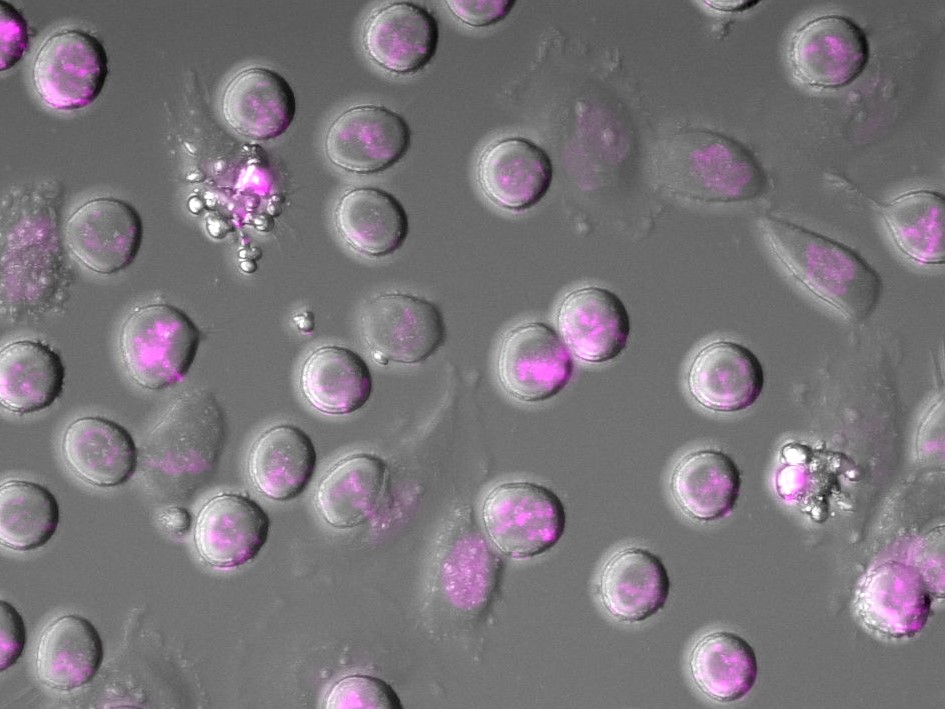
Image: Microscopy analysis of cancer cells dying in an experiment mimicking chemotherapy in cell culture. Cells are in the process of dying. DNA is stained in magenta. (Credit: Dr Christian Zierhut)
Everything from sunlight to ‘copy and paste’ errors in cell growth and division leaves a mark on our DNA. This everyday damage is manageable because of our cells’ built-in repair system known as the DNA damage response. However, if something goes wrong in this complex signalling system, or too much damage builds up, it can lead to cancer.
In the 1960s, our scientists uncovered the first conclusive evidence that DNA damage was the fundamental cause of cancer. Over the decades, we have created treatments targeting cancer cells with faulty repair systems, such as those with mutations in the BRCA breast cancer genes.
However, the cell’s DNA damage response is hugely complex and has many moving and interconnected parts – making it difficult to target. Our scientists are exploring the underlying biology of the DNA damage response system and its interconnected pathways to open up new ways of attacking cancer.
We provided the first conclusive evidence that the fundamental cause of cancer is damage to our DNA. Learn how our scientists are exploiting the DNA damage response pathway.
DNA damage repair
Exploiting cancer’s vulnerability

Image: Dr Christian Zierhut (centre) leads the Genome Stability and Innate Immunity Team at the ICR.
Dr Christian Zierhut leads our Genome Stability and Innate Immunity Team. His team studies how defects in the systems for detecting and repairing DNA damage can have major consequences for cells.
He says: “In cancer, the DNA of cells becomes unstable and prone to damage. This instability can allow cancer cells to adapt, evolve and become more aggressive. But it also leaves cancer more vulnerable in ways we can exploit.”
Dr Zierhut’s team is currently focusing on a pathway connected to the response to DNA damage which can trigger a type of immune mechanism called innate immunity. Unlike acquired immunity, in which the body produces antibodies in response to infection, innate immunity keeps cells on constant alert so they can recognise and combat viruses.
The team is interested in the cGAS pathway, which is typically dormant in our cells. When activated, the pathway should allow our cells to better detect cancer cells. However, if it’s turned on at the wrong time or too often it can instead be used by cancer cells to grow and spread, or even suppress the immune system.
Dr Zierhut says: “This pathway is like a double-edged sword. In principle, it should help the immune system eliminate cancer – but cancer cells can undergo changes to either silence it or use it to their own ends. We need to find ways of wielding this double-edged sword against cancer.”
Recreating a pathway in a test tube
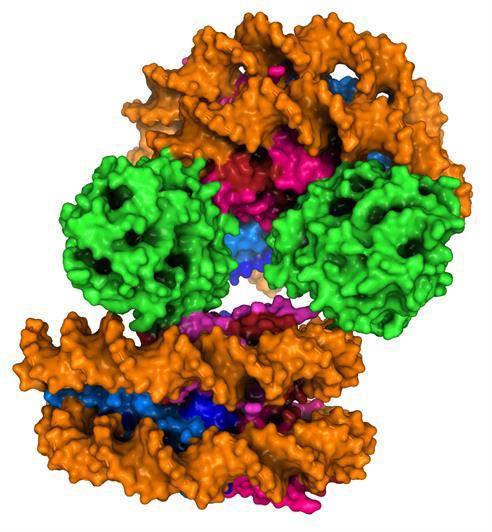
Image: cGAS nucleosome structure
The key to harnessing this pathway is by studying its biology. We need to understand how it works on a mechanistic level and why each part can contribute to cancer cell growth or death in different situations.
To study the cGAS pathway, Dr Zierhut’s team recreates its biochemistry in the lab. The team studies the role of individual components of the system by either purifying them in a test tube or by using extract from cells. The researchers have also designed cells that light up with fluorescence to show what is happening when the system is activated.
From the lab to the clinic
Ultimately, building our understanding of the basic mechanics of a cell is crucial in helping patients. Dr Zierhut’s team is currently collaborating with scientists and clinicians specialising in genetics, epidemiology, radiotherapy and immunotherapy. When his team has a promising lab result, they can see whether it aligns with patterns in patient data. Or they can learn from the experience in the clinic in planning new lines of inquiry for experiments. This overlap is especially promising in areas such as radiotherapy, which can cause DNA damage, and immunotherapy, which relies on activating cells’ immune system.Dr Zierhut says: “We are trying to understand all the layers of complexity from biochemistry to cell biology and all the way up to patients. If we can understand how this pathway works, we could create tools to manipulate the system and to start using cells’ natural defences against cancer.”
This feature originally appeared in Search magazine. Read past issues or subscribe to our supporter newsletter Search.