September 2021
AI identifies new drug combination for childhood brain cancer
Image: Human paediatric brain tumour cells. Credit: Valeria Molinari, Louise Howell, Maria Vinci, Katy Taylor and Chris Jones via the Wellcome Collection. Licence: CC BY NC.
Scientists have used artificial intelligence tools to successfully propose a new combination of drugs for use against diffuse intrinsic pontine glioma (DIPG), an incurable childhood brain cancer.
The team, led by Professor Chris Jones, Professor of Paediatric Brain Tumour Biology at the ICR, worked with the company BenevolentAI – which has built a leading AI drug discovery platform and its own pipeline of drug discovery programmes.
The researchers identified drugs that could be used to target mutations in a gene called ACVR1 in DIPG. Although a quarter of children with DIPG have an ACVR1 mutation, there is currently no treatment that targets it that is approved for use. Vandetanib is a drug that acts against ACVR1 but has difficulty getting past the blood-brain barrier.
The study found that combining vandetanib with a drug called everolimus could enhance its capacity to pass into the brain to treat the cancer. The proposed combination has proven effective when tested in mice, extending survival by up to 14 per cent, and has already been tested in a small cohort of children.
October 2021
Gut bacteria can drive prostate cancer growth and treatment resistance
Image: Section of a mouse gut. Credit: Kevin Mackenzie, University of Aberdeen.
Scientists led by Professor Johann de Bono, Professor of Experimental Cancer Medicine at the ICR, have found that common gut bacteria can fuel the growth of prostate cancers and allow them to evade the effects of treatment.
Hormone therapy, a staple treatment for prostate cancer, limits tumour growth by reducing androgen levels. Combining findings from studies in mice and patient data, the researchers found that the resulting low androgen levels can drive the expansion of gut bacteria. These provide an alternative source of growth-promoting androgens, sustaining prostate cancer growth. After analysis of stool samples of people with prostate cancer, the bacteria Ruminococcus was identified as driving resistance, whereas Prevotella stercorea was associated with favourable clinical outcomes.
Using organoids derived from men with prostate cancer, the team identified bacterial 'fingerprints' that could identify those at high risk of developing resistance to hormone therapy. They hope those vulnerable to developing resistance could benefit from microbiome manipulating strategies – for example undergoing a faecal transplant or taking a yogurt drink enriched with favourable bacteria.
ICR researchers involved in this paper: Penny Flohr, Joanne Hunt, Antje Neeb, Lorenzo Buroni, Christina Guo, Jonathan Welti, Johann de Bono.
October 2021
Targeted prostate cancer screening could benefit men with inherited cancer syndrome

Men who inherit an increased risk of cancer through Lynch syndrome could benefit from regular prostate-specific antigen (PSA) testing from age 40 to detect early signs of prostate cancer, say scientists led by Professor Ros Eeles, Professor of Oncogenetics at the ICR.
The research, which is part of the international IMPACT study, found that annual PSA testing could pick up cases of prostate cancer up to eight times as often in men with genetic hallmarks of Lynch syndrome, such as faults in repair genes like MLH2 and MSH6, than in those without.
While PSA screening isn't recommended for the general population because of concerns about over-diagnosis and over-treatment, this study, shows it could carry more promise for those with Lynch syndrome. Many of the cancer cases in men with the disorder were 'clinically significant', suggesting targeted screening has the potential to save lives. Identifying those with Lynch syndrome could also guide their treatment as increasing evidence suggests that this group could respond particularly well to immunotherapies.
January 2022
Uncovering how cancers resist precision treatment

Image: Counting pills Credit: Jan Cheblik.
Scientists led by Professor Chris Lord, Professor of Cancer Genomics at the ICR, have revealed how cancer can resist PARP inhibitors – a type of precision medicine used to treat thousands of patients worldwide for ovarian, breast, prostate, and pancreatic cancer.
PARP inhibitors target a protein used by cells to repair DNA called PARP1 – locking it in place on the DNA to render it inactive. Some cancer cells are particularly vulnerable to PARP inhibitors because they also have other weaknesses in their DNA repair machinery, so the drugs kill them effectively. However, PARP inhibitors don't work for everyone, and it is estimated that over 40 per cent of eligible patients don't respond to them.
The team found that some cancer cells dodge the effects of PARP inhibitors by removing the PARP proteins that get trapped on their DNA. A small molecule called p97 could play a crucial role in this, saving cancer cells from destruction. Therefore, blocking p97 could open up new ways to treat cancer.
March 2022
Test can personalise use of immunotherapy and chemotherapy for head and neck cancer
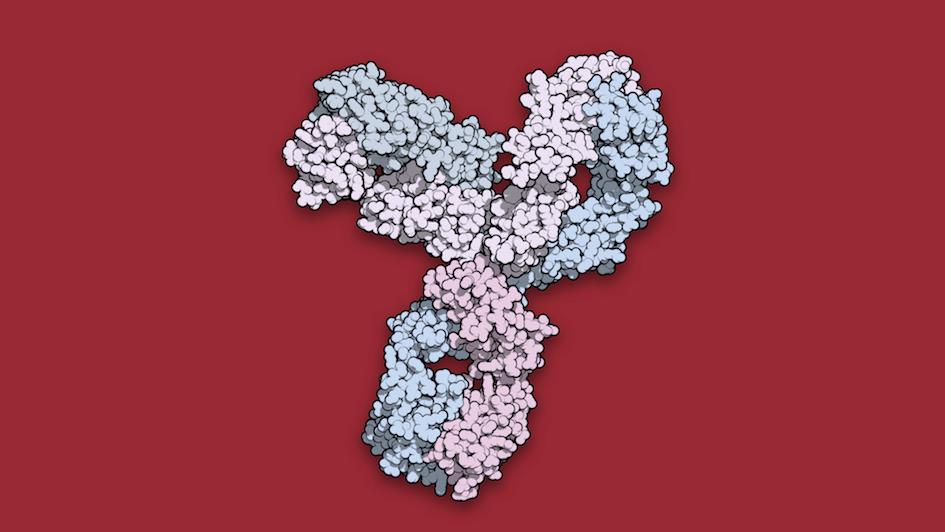
Image: Space-filling model of immunotherapy pembrolizumab.
Matching cancer patients to treatments based on their levels of a key immune protein may allow doctors to select those who would benefit the most from a combination of immunotherapy and chemotherapy.
Researchers at the ICR and The Royal Marsden showed that patients with head and neck cancer enrolled in the major KEYNOTE-048 trial benefitted from different treatments depending on the results of a test, which measured levels of the protein PD-L1 in tumours and on surrounding cells. Patients with low levels of PD-L1 are highly unlikely to benefit from the immunotherapy drug pembrolizumab alone, and should receive chemotherapy alone or chemotherapy plus the drug cetuximab. But patients with moderate PD-L1 levels may benefit most from a pembrolizumab and chemotherapy combination, and those with high PD-L1 levels may benefit most from pembrolizumab alone.
The researchers, led by Professor Kevin Harrington, Professor of Biological Therapies at the ICR, hope that use of the test will be adopted in guidelines as a way to personalise patients' treatment by selecting immunotherapy, chemotherapy or the combination of the two depending on PD-L1 levels.
April 2022
AI test could predict cancer drug combinations in less than two days

Scientists led by Professor Udai Banerji, Professor of Molecular Cancer Pharmacology at the ICR, have created a prototype test using artificial intelligence that can predict which drug combinations are likely to work for cancer patients in as little as 24 to 48 hours.
The cutting-edge test uses AI to analyse large-scale protein data from tumour samples and can predict patients' response to drugs more accurately than is currently possible. The team tested the new technique on individual cancer cells in the lab and tumour cells isolated from lung fluid in people with non-small cell lung cancer.
As well as successfully identifying drug combinations that have previously been shown to have promise, the test also found possible new combinations, such as using vemurafenib together with capivasertib.
This is the first prototype test that can offer personalised predictions of which drug combinations are likely to work in different individuals. Researchers at the ICR believe the new technology could be crucial in overcoming cancer evolution and treatment resistance by allowing doctors to analyse how drugs work in combination.
June 2022
A mechanism controlling spread of pancreatic cancer
Image: Pancreatic cancer cell. Credit: Anne Weston, Francis Crick Institute.
Scientists led by Professor Axel Behrens, Professor of Stem Cell Biology at the ICR, have identified a new mechanism that controls the growth of dangerous pancreatic cancer cells. The team found that triggering the mechanism can lead to more aggressive disease - but also crucially that the fate of these cancer cells can be reversed.
The findings show that a protein called GREM1 controls genetic diversity in pancreatic cancers and plays a vital role in regulating their behaviour. Deactivating the GREM1 gene in mice switched pancreatic cancer cells into a more dangerous and invasive cell type – but in cells where GREM1 was highly active there was a near-complete reversal back to their less aggressive, more manageable state. This novel finding could open opportunities to discover future treatments for pancreatic cancer, which has the lowest survival rate of all common cancers.
June 2022
New combination therapy destroys head and neck tumours
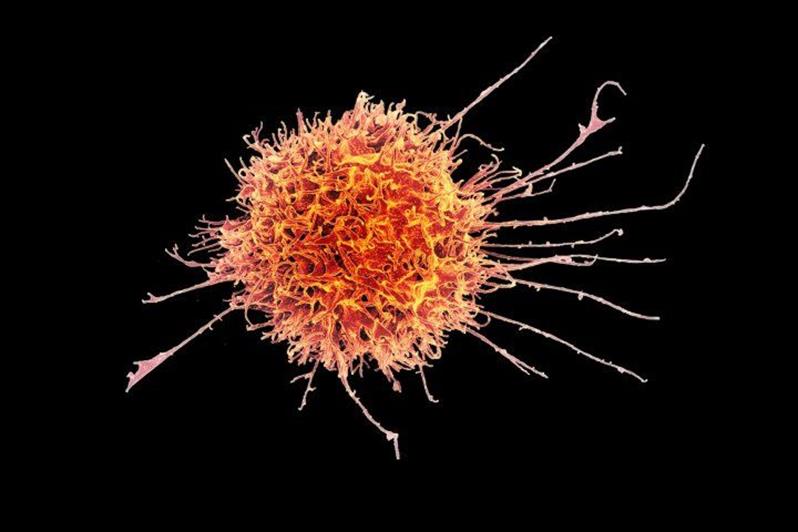
Image: Colourised scanning electron micrograph of a natural killer cell from a human donor. Credit: National Institutes of Allergy and Infectious Diseases.
A treatment combination of radiotherapy, immunotherapy and a DNA repair inhibitor drug is highly effective at suppressing the growth of head and neck tumours, new research has found. The new therapy works by activating natural killer cells, a type of white blood cell that can kill cancer cells directly.
Researchers led by Professor Kevin Harrington, Head of the ICR's Division of Radiotherapy and Imaging, showed the benefits of the new combination treatment in mice. When mice with head and neck cancer received radiotherapy plus a drug that inhibits ATR – a key protein in DNA damage repair – they survived longer than those that received either treatment alone. Adding in immunotherapy to boost the anti-tumour activity of natural killer cells created an even more effective combination – with some mice having their tumours completely eradicated.
Only around 15-20 per cent of patients with head and neck cancer respond to current immunotherapies. However, the new strategy to boost the effectiveness of natural killer cells could considerably expand the number of patients with these cancers who benefit.
July 2022
'Junk' DNA could lead to cancer by stopping copying of DNA
Scientists led by Dr Gideon Coster, Team Leader in Genome Replication at the ICR, have shown that repetitive non-coding 'junk' DNA may get in the way of the replication and repair of our genome, potentially allowing mutations to accumulate.
By recreating the entire process of DNA replication in a test tube, the researchers found that when DNA replication machinery encounters repetitive DNA it sometimes fails to copy both strands successfully. This error could cause the replication to stall, in a manner similar to that induced by DNA damage.
DNA damage and the downstream genome instability are known to promote cancer formation and progression, so the research strengthens the link between junk DNA and cancer. The scientists hope that having an improved understanding of how DNA replication can go wrong might lead to new ways of treating the disease, in addition to better diagnosis and monitoring of some cancers.
July 2022
Targeting cancer-supporting cells boosts immunotherapy response in insensitive tumours
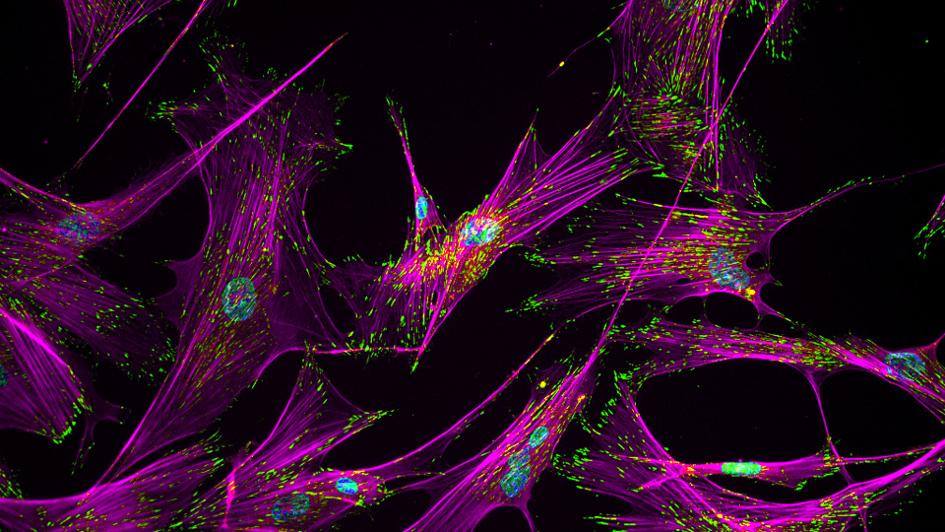
Image: Fibroblasts. Credit: Ankur Singh, Georgia Tech.
Removing a receptor from cancer-associated fibroblasts (CAFs), a feature of the tumour microenvironment, improves the response to immunotherapy of cancers that were previously insensitive to it.
The study, led by Professor Clare Isacke, Head of the Division of Breast Cancer Research at the ICR, used parallel mouse models with CAF-rich and CAF-poor tumours to find that cancers with a high number of CAFs in their microenvironment contained fewer CD8+ T cells. This 'immune cold' ecosystem meant that immunotherapies were less effective.
The team also found the receptor Endo180, which is upregulated on CAFs, was connected to this relationship between the fibroblasts and reduced CD8+ infiltration. When Endo180 is knocked out, mice had more CD8+ T cells in their tumours and a better response to immunotherapy.
Breast and pancreatic cancers are two examples that are often CAF-rich, and they are also resistant to immunotherapy. This research indicates that CAFs, and more specifically the Endo180 receptor, could make good targets to enhance the response of immunotherapy in those for whom it doesn't currently work.