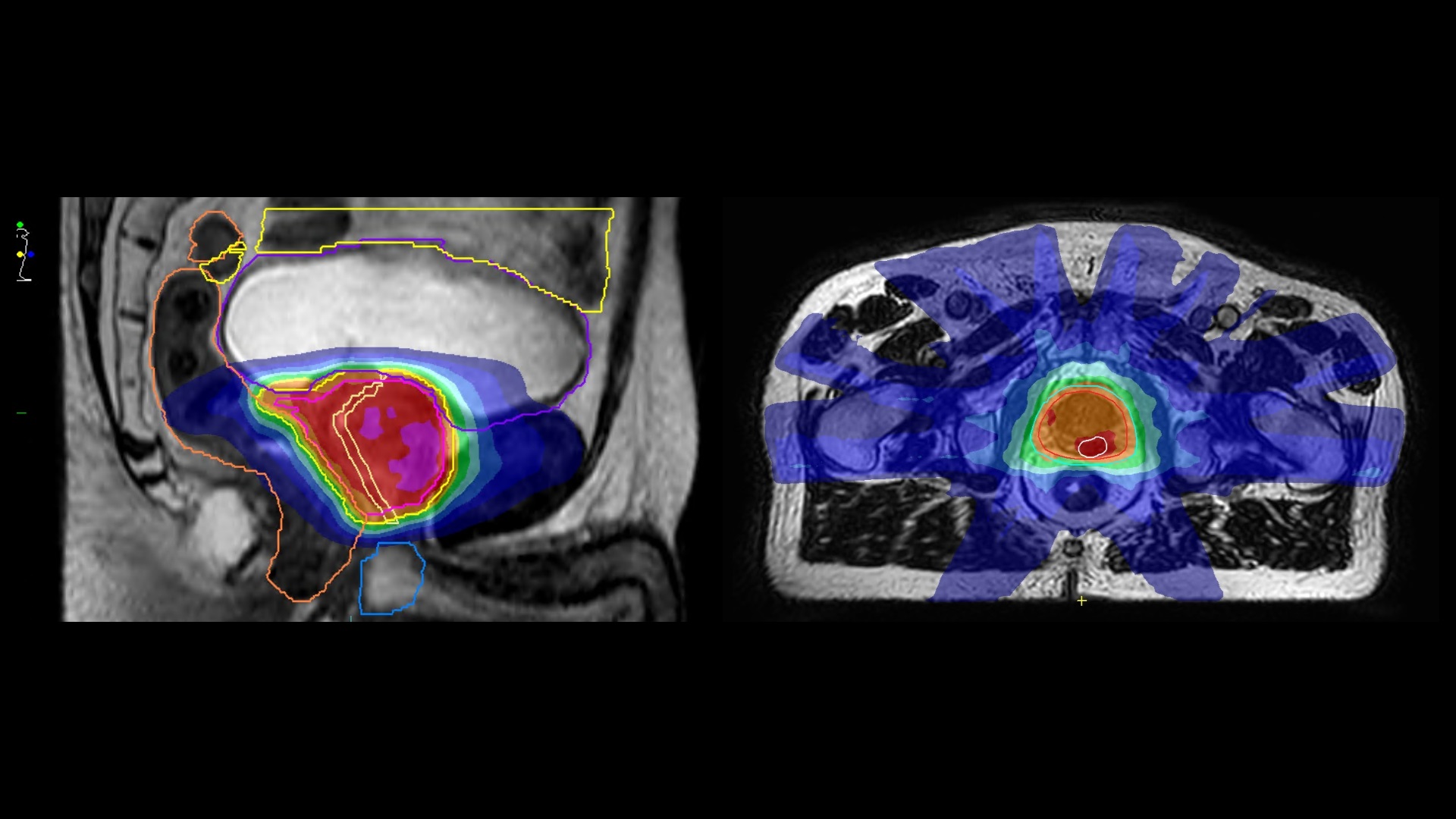
Radiation Research Centre of Excellence (RadNet)
The Radiation Research Centre of Excellence (RadNet-CoE) at The Institute of Cancer Research and The Royal Marsden brings together preeminent leaders in radiotherapy, immunotherapy, cancer biology, and targeted drug therapies to foster ground-breaking collaborations to pioneer innovative therapeutic approaches, with the goal of transforming cancer care on a global scale.

How we research at this centre
The RadNet-CoE at the ICR and The Royal Marsden was established in 2019 with the central aim of bridging discovery, translational, and clinical research to benefit patients directly. Positioned at the forefront of radiation-related research, the RadNet-CoE leverages the intersection of world-class laboratory scientists specialising in DNA repair, cell death, immune response, and cancer evolution, alongside clinicians, radiographers, physicists, and research methodologists who design and conduct clinical trials to advance global cancer care.
In 2024, the RadNet-CoE successfully secured a second, uplifted round of funding from Cancer Research UK, which will support:
- The enhancement of our cutting-edge research infrastructure to drive innovative radiation research
- The development of the next generation of radiation researchers through targeted training initiatives.
This second phase of the RadNet-CoE, led by Professor Kevin Harrington and Dr Alison Tree, focuses on improving cancer treatment outcomes by gaining a deeper understanding of how radiotherapy can optimally modulate key biological processes at cellular, microenvironmental, and organismal levels.
Video: Dr Alison Tree and Professor Kevin Harrington talk about the future direction of RadNet's research at the ICR and The Royal Marsden.
Research themes
The Centre’s research is structured around four critical themes:
Led by Christian Zierhut and Ben O’Leary
In Theme 1, we aim to explore strategies to disrupt cancer’s DNA damage repair mechanisms induced by radiation, thereby maximising the cytotoxic effects of radiotherapy and enhancing its ability to activate immune responses. Building on our existing research, we will investigate how to convert immunologically silent forms of apoptosis into immune-stimulatory cell death, thereby improving cure rates. Additionally, we will examine the role of intratumoural oxygen levels (redox state) in influencing cell death pathways and immune-mediated cytotoxicity. Our work will involve preclinical and translational research to better understand this evolutionary process and identify potential vulnerabilities in cancer cells for novel therapeutic approaches.
Led by Anna Wilkins and Alan Melcher
Theme 2 will focus on strategies to enhance the tumour microenvironment (TME) in response to signals from dying cancer cells. Specifically, we will explore how radiotherapy combined with other therapeutic agents, such as DNA repair inhibitors, pattern-recognition receptor agonists, and agents that promote immunogenic cell death, can modulate the TME. We will profile treatment-induced changes within the TME and expand our efforts in clinical sample collection, processing, and analysis using cutting-edge platforms. This will allow us to validate preclinical findings and generate new hypotheses that could further optimise cancer therapies.
Led by Uwe Oelfke and Magnus Dillon
In this theme, we will investigate emerging radiation delivery technologies that offer exciting opportunities to enhance the biological effectiveness of radiotherapy, particularly FLASH and spatially-modulated radiotherapy (SMRT), including microbeam, minibeam, and GRID techniques. Our Centre houses the world’s first FLASH-enabled small animal radiotherapy research platform (SARRP), capable of delivering ultra-high dose rates up to 180 Gy/s, with both conventional and spatially-modulated beam distributions. This unique capability will enable us to contribute valuable insights into the fundamental mechanisms of these next-generation radiotherapy technologies. Additionally, we will explore the potential of the nanoparticulate radioenhancer, NBTXR3, in combination with FLASH and SMRT techniques.
Led by Alison Tree and Anna Kirby
In Theme 4, we will leverage clinical trial samples and biobank collections from ongoing and upcoming clinical studies to validate and refine the findings across the other three themes. Our goal is to ensure a seamless transition of research from the laboratory to clinical practice. Using this extensive repository, we will conduct “reverse translation,” integrating patient data with molecular analyses to link fundamental scientific discoveries with tangible patient outcomes. This approach will allow us continuously to refine and optimise therapeutic strategies in real-world clinical settings.
Future directions
Professor Kevin Harrington, Head of the Division of Radiotherapy and Imaging at the ICR and Consultant Clinical Oncologist at The Royal Marsden, said:
“The ICR has been leading the way for radiation-related research in the UK – in terms of the fundamental discoveries, the translational opportunities and the clinical implementation of developments. Our research has led to many of the most pivotal radiation trials, improving therapeutic options over the last three decades.
“Along with the other main bodies within the UK that lead on this research, we can ensure that we steer the collective direction of research of the UK’s research community. Of course, we always do so with a view to making sure that patients both within the UK and globally have access to state-of-the-art treatments for their cancers. This funding from RadNet cements our role in continuing that legacy and will allow us to cure more patients while causing less damage.”
Dr Alison Tree, Consultant Clinical Oncologist at The Royal Marsden and Honorary Faculty in the Division of Radiotherapy and Imaging at the ICR, said:
“Historically, radiation research has been underfunded despite being integral to cancer treatment. With the support of CRUK, we can enhance our research efforts, ultimately improving patient outcomes and ensuring that radiation therapy receives the attention it deserves in the fight against cancer.
“There is a significant lack of knowledge among the general population about what radiation therapy is and how helpful it can be. Modern radiotherapy is a gentle yet powerful treatment that is responsible for almost half of cures against cancer. The support from RadNet enables the ICR and The Royal Marsden to continue as leaders in this field, creating cutting-edge, patient-focused therapies that yield tangible results.”
News and discoveries
The HERMES trial, led by Dr Alison Tree and Professor Emma Hall, is the first randomised trial in the world to test just two treatments of radiotherapy on the MR linac. The trial has embedded translational work to examine the immune consequences of very high dose per fraction. The interim analysis, published last year shows that the treatment, designed to cure prostate cancer, has lower levels of side effects than expected. The trial has now completed and full clinical analysis is expected to be presented in 2024.

The MR linac team, including doctors, radiographers and one of our patient advocates, have been interviewed as part of a 6-part podcast series on our MR linac research, as part of the MR linac consortium.
Click here to hear from one of the patients partnering with us in research.
Amy Burley, a PhD student in the Stromal Radiobiology group led by Dr. Anna Wilkins, was recently awarded the prize for the best oral presentation at the 6th Bladder Cancer Translational Research Meeting.
Amy’s abstract was selected as one of the top three submissions and she was invited to give a talk summarising her work looking at strategies to predict radiotherapy recurrence in bladder cancer.
Looking at the genetic profiles of patients treated with radiotherapy, Amy has found that an enrichment for signatures linked to fibrotic cells in the tumour microenvironment was associated with significantly worse outcomes.
The audience were impressed by Amy’s observations and were encouraged by her plans to correlate these findings with multiplexed imaging data (see image below).
Amy’s analysis helps us to understand the mechanisms that drive radiotherapy resistance in bladder cancer and may identify new opportunities to improve responses to this treatment.
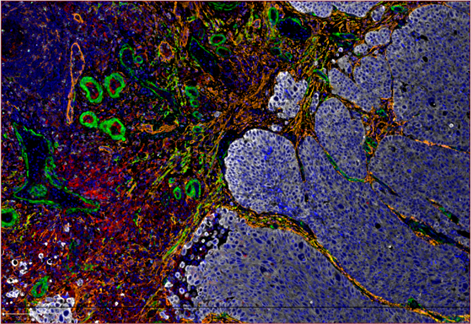
A multiplexed image of the tumour microenvironment in a bladder cancer sample. Tumour cells are shown in white and diverse populations of fibrotic cells are shown in orange, red, yellow and green.
Ceralasertib showed promise for patients no longer responding to current cancer treatments in an early clinical trial. Given on its own, the drug stabilised the growth of tumours in more than half of patients who received it, with one patient seeing benefits for more than five years.
The ICR’s Genome Stability and Innate Immunity Group, headed by Dr Christian Zierhut, studies how the genotoxic stress that is associated with cancer can be exploited to promote immune responses. The group has a special interest in radiotherapy, and most of the group’s work is closely associated with the ICR/RM Radiation Research Centre of Excellence. Recently, two new projects were funded for work carried out by the group, both of which build on, and expand, previous insight generated in association with the Centre of Excellence. The first of these is funded by a project grant from Breast Cancer Now. It will make use of novel single-cell analysis tools that the group previously developed to investigate how the DNA repair-defective mutations that can be associated with breast cancer impact on cellular immune responses. Initially, this research will focus on a class of anti-cancer drugs that are known as PARP inhibitors, and subsequently, the group will also apply their efforts on investigating radiotherapy-related responses.
The second project is funded by the Japan Agency for Medical Research and Development (AMED), and allows Dr Tomoya Kujirai from the University of Tokyo to carry out a sabbatical in Dr Zierhut’s group at the ICR. In this project, the researchers will build on previous collaborative work, and generate a detailed biochemical picture of how DNA-damage induced immune responses are controlled. Altogether, the conclusions drawn from these projects are expected to lead to new approaches for stratifying patients for radiotherapy, and to new co-treatments that can make radiotherapy more effective.
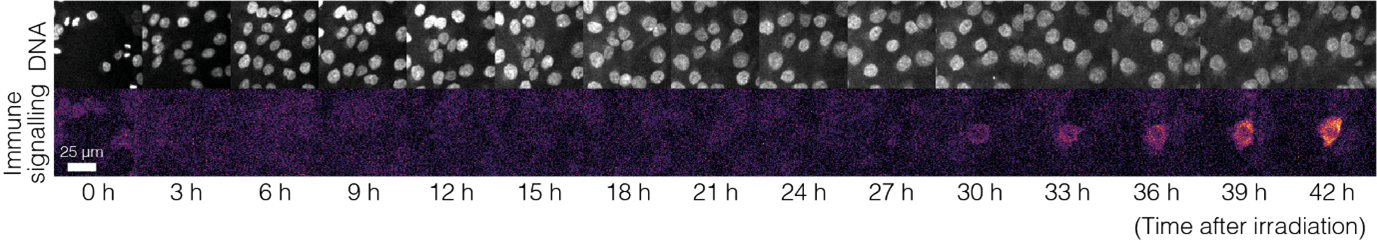
Credit: Vicente Lebrec/ICR. A cell undergoing radiation-induced immune responses
In a newly published review article, entitled ‘Potential cGAS-STING pathway functions in DNA damage responses, DNA replication and DNA repair’, Dr Zierhut, who leads the Genome Stability and Innate Immunity Group at the ICR, summarises the available evidence on innate immune regulation of genome stability, and suggests solutions to potentially conflicting data. The full paper is available here