Centre for Genome Stability
at the ICR and The Royal Marsden
Centre for Genome Stability (CGS) is a unique multidisciplinary programme bringing together experts from across the ICR and The Royal Marsden who work on DNA-damage response and genome instability research with clinical experts in the treatment of cancer.
How we research at this centre
Our world-leading centre of excellence aims to understand genetic, epigenetic and proteomic changes to genomic stability in the development of cancer in order to develop biomarkers and therapeutic targets for improved cancer treatment. This will allow us to apply multidisciplinary approaches to address important research questions, thus accelerating discoveries into the causes of tumorigenesis and delivering major clinical impacts, both nationally and internationally.
We aim to harness the breadth of expertise in genomic stability at the ICR to develop new and better therapies against cancer to make the most meaningful impact in clinics.
The centre will establish a single-domain antibody screening facility to generate DNA-repair related biomarker detection and intracellular antibody protein perturbation tools.
The Centre's main themes are as follows:
Molecular Mechanisms of Genome Stability Maintenance
Maintenance of genome stability is accomplished throughout the cell cycle by a complex network of proteins referred to as the DNA-damage response (DDR) pathways. Mutations in DDR factors are associated with elevated cancer predisposition. Understanding this will allow us to target tumour-specific DDR dependencies for improved cancer treatments.
Understanding Replication Stress Responses
Cells control DNA replication with the highest possible fidelity in order to prevent catastrophic changes to their genome. Understanding how DNA replication and repair machineries function and how these processes differ in cancer cells could provide new prognostic tools and therapeutic targets.
Integrative Systems Biology of Cancer and Ageing
We will develop tools to interrogate large data sets of genetic, genomic and proteomic information to generate hypotheses for experimental analysis, with the ultimate goal of understanding the changes that occur to the genomes and proteomes of cells as they transition to a cancerous or aged state.
Genome Instability and Cancer Therapeutics
New dependencies within the DDR pathways may represent targetable vulnerabilities in cancer cells, with a major focus on synthetic-lethal interactions and over-reliance on DDR pathways allowing for selective killing of cancer cells.
We want to accelerate the translation and delivery of our research.
As one of the world’s most influential cancer research institutes, we discover more new cancer drugs than any other academic centre in the world. We have made game-changing discoveries that revolutionised the way cancer is studied and treated.
Work with us in the Centre for Genome Stability to develop and commercialise our novel discoveries to defeat cancer. Contact us at [email protected] to work with us.
Internal collaborative opportunities
The Centre supports activities to bring together genome stability expertise across the ICR and The Royal Marsden in order to support and develop new collaborative opportunities.
Centre short talk series
Participate in our series of short talks and hear from colleagues. Next event is expected to take place in October 2024. Further details TBC.
Single-domain Antibody Facility
We have established our Single-domain Antibody Facility with ongoing pilot studies. There will be a call for screening projects.
Requirements to access the facility
Protein/antigen concentration of 500µg – 1mg, with objective of antibody for advice on tag (i.e. denatured/native form of protein). Final validation to be undertaken by research teams due to limited capacity within the facility. If you are interested to find out more, please contact Dr Divya Duscharla at [email protected].
Clinical Research Fellowships
Colleagues across the ICR and The Royal Marsden can apply to appoint Clinical Research Fellows (3-year PhD), addressing a reverse translation topic (bed-to-benchside) and involving clinical and fundamental research labs.
We are now accepting outline project proposals from potential supervisors for the next round of Clinical Research Fellowships for 2025/26 entry.
Please contact [email protected] to express your interest or if you have any questions.
Our researchers at this centre
Fundamental research
Biography
Professor Chris Lord is Deputy Head of Division, Group Leader of the CRUK Gene Function Laboratory and Professor of Cancer Genomics in the Breast Cancer Now Toby Robins Research Centre at The Institute of Cancer Research, London. Much of his work focuses on exploiting genetic concepts such as synthetic lethality to identify new approaches to treating cancer and to understand the variable effectiveness of existing treatments.
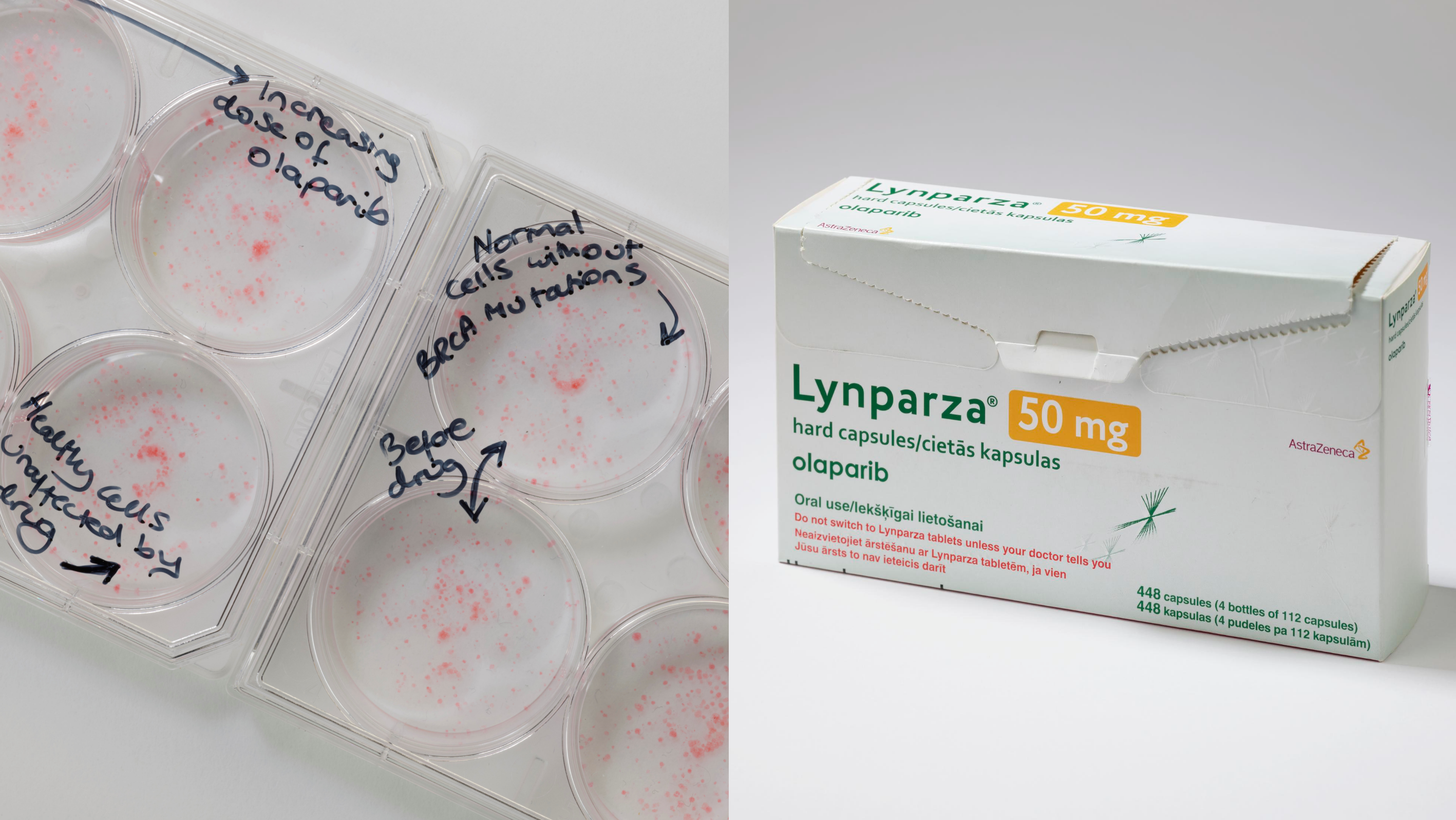
Chris started his career with PhD training in disease genetics with John Todd and Richard Gardner at the University of Oxford before carrying out a Postdoctoral Fellowship with Todd at the University of Cambridge. Chris joined the ICR London as a Postdoctoral Fellow with Alan Ashworth in 2000, where he was joint first author on a paper describing the synthetic lethal interaction between BRCA-tumour suppressor genes and PARP inhibitors (Nature 2005), observations that eventually led to the use of these drugs for the treatment of breast, ovarian, prostate and pancreatic cancers.
Later, Chris exploited high-throughput genetic perturbation screens to understand a variety of cancer-related phenotypes including drug sensitivity/resistance and the identification of novel therapeutic targets (e.g. Cancer Cell 2008, Cancer Discov. 2011), a number of which are now being investigated as part of new drug development programmes. Chris has also used multiple approaches to uncover and/or understand clinically-relevant mechanisms of resistance to DNA repair inhibitors (e.g. Nat Commun. 2018, Cancer Discov. 2020) and to identify novel synthetic lethal approaches that target hard-to-treat cancers, including those with ARID1A, Rb or E-cadherin defects (e.g. Cancer Discov. 2018).
More recently, Chris has focused on using high-throughput genetic perturbation screens to understand the principles that govern the robustness of synthetic lethal interactions (e.g. Elife 2020). The impact of the work led by Lord is demonstrated by the multiple biomarkers of drug sensitivity/resistance and novel synthetic lethal approaches to the treatment of ARID1A, Rb or E-cadherin defective cancers now being assessed in clinical trials.
Disclosure information. CJL is an inventor on patents describing the use of PARP inhibitors and stands to gain from their development as part of the ICR’s “Rewards to Inventors” scheme. CJL has received research funding, honoraria, consultancy or advisory board payments from the following: Sun Pharma, Vertex Ltd., 3rd Rock, Horizon Discovery, Gerson Lehrman Group, Guidepoint, Abingworth, Astra Zeneca, Merck KGaA, Artios, Ono Pharma, Tango Therapeutics and LEK. CJL owns shares in Tango Therapeutics.
Chris Lord is a member of the Cancer Research UK Convergence Science Centre, which brings together leading researchers in engineering, physical sciences, life sciences and medicine to develop innovative ways to address challenges in cancer.

.png?sfvrsn=38d02ad6_1)