April 2020
One-week course of radiotherapy could benefit women with breast cancer
Image: radiotherapy machine used in the treatment of many kinds of cancer, including breast cancer
A trial co-led by ICR researchers found that a one-week course of radiotherapy in fewer but larger daily doses was as safe and effective as standard three-week treatment for women following surgery for early-stage breast cancer.
The pioneering study involving more than 4,000 patients from 97 NHS hospitals evaluated the effectiveness of two different radiotherapy regimens each delivered over five days in one week, compared with standard radiotherapy delivered in 15 doses over three weeks.
The results of the FAST-Forward trial were published in The Lancet, and could spare patients hospital appointments and save NHS resources.
Study co-leader Professor Judith Bliss, Professor of Clinical Trials at The Institute of Cancer Research, London, and Director of its Clinical Trials and Statistics Unit, said:
“We’re always looking for ways to refine and enhance cancer treatment so we can make it more effective and improve the experience for patients.
“We expect these findings will be incorporated into breast cancer treatment guidelines around the world and we’re already seeing NHS hospitals wanting to move to the five-dose schedule because of the challenges they’re facing during the coronavirus pandemic.”
The trial was funded by the National Institute for Health Research (NIHR).
June 2020
‘Drugs and diet’ treatment could target cancer’s reliance on fat
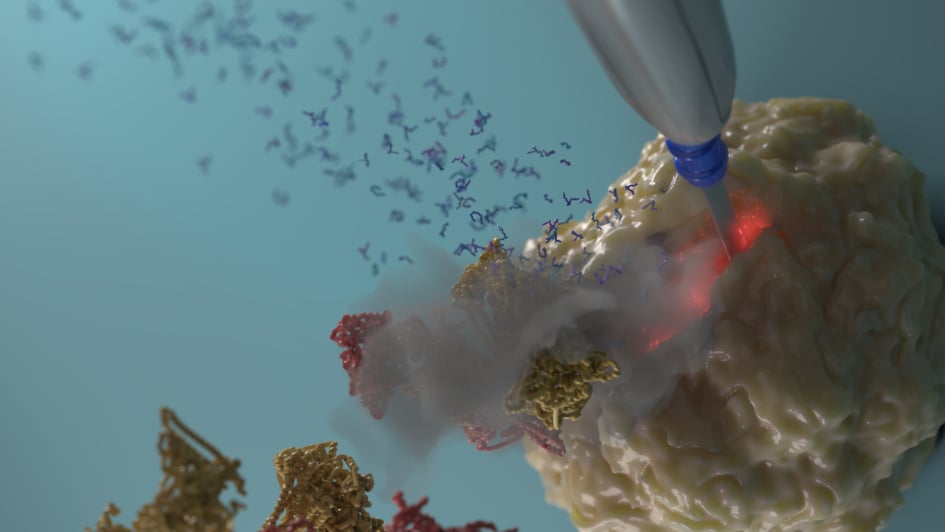
Image: iKnife cutting into a tumour. Credit: Jeroen Claus, Phospho Biomedical Animation
Cancers are often heavily reliant on breaking down fats for their growth and spread, and could be treated by a highly innovative combination of new drugs and dietary changes, ICR scientists have concluded.
The researchers used a high-tech surgical ‘iKnife’ to analyse vaporised cancer tissue – and identified a metabolic weakness in cancer that could be amenable to a new metabolic treatment.
They found that targeting cancer’s ability to process fat using a new class of drugs could halt tumour growth in mice, but only when combined with a diet free of fats.
The study, published in the prestigious journal Cell, opens up the possibility of ‘drugs and diet’ cancer treatments – combining precision medicine and dietary changes.
Study leader Dr George Poulogiannis, Leader of the Signalling and Cancer Metabolism Team, said:
“We have shown that analysing cancer’s metabolic fingerprint can be a key tool in understanding, diagnosing and treating the disease. Our research raises the prospect that in future we could analyse cancer’s metabolic fingerprint while patients are on the operating table using the iKnife, and where appropriate match them to a combined drug and diet treatment.”
The research was funded by the ICR and Cancer Grand Challenges.
May 2020
Study gives new insights into how DNA is organised

Image: Shadow of a DNA double helix on coloured DNA sequencing output. Credit: Peter Artymiuk, Wellcome Collection, CC BY 4.0
ICR scientists have uncovered new information about vital structures inside cells which are responsible for organising our DNA.
Using state-of-the-art imaging techniques, the team were able to look at two critical structures responsible for condensing DNA into chromosomes, called condensin I and condensin II.
The findings could have major implications for understanding how cancer develops, since these structures often malfunction in cancer cells, leading to harmful mutations in the DNA.
The study, carried out by our scientists in conjunction with a team at Columbia University in the US, was published in the journal Molecular Cell.
Co-leading author Dr Erin Cutts, Post-Doctoral Training Fellow in Structural Biology at the ICR, said:
“In this work we could directly see how individual human condensin I and II molecules were structured, allowing us new insights into how condensin works.”
Study co-leader Professor Alessandro Vannini, Team Leader in Structural Biology, said:
“These two complexes are often altered in many different types of cancer, so understanding the structure and function could help with future work to create new treatments.”
The research was funded by the Wellcome Trust, Cancer Research UK and the ICR.
September 2020
Breast cancer drug could transform prostate cancer treatment
Image: Prostate cancer cells. Credit: Professor Johann de Bono and Mateus Crespo, The ICR
ICR researchers found that olaparib, a drug approved for breast and ovarian cancer, was more effective than targeted hormone therapies at keeping cancer in check in some men with prostate cancer.
Olaparib targets cancer cells with faulty systems for repairing DNA – taking advantage of an Achilles heel in these cells while sparing healthy cells. It was researchers at the ICR who discovered how to genetically target the drug.
Olaparib blocked prostate cancer growth more effectively than abiraterone and enzalutamide in men with advanced disease who had defects in one or more of 15 genes involved in DNA repair.
Men with faulty BRCA1, BRCA2 or ATM genes benefited the most from receiving olaparib – with their disease taking 7.4 months before it progressed, compared with 3.6 months for those on enzalutamide or abiraterone.
The trial, PROfound, highlights the importance of genetic testing in men with prostate cancer.
Study co-leader Professor Johann de Bono, Professor of Experimental Cancer Medicine at the ICR and Consultant Medical Oncologist at The Royal Marsden, said:
“This study is a powerful demonstration of the potential of precision medicine to transform the landscape for patients with the most common of male cancers.”
The study, funded by AstraZeneca, was published in the New England Journal of Medicine.
April 2020
Babies with brain tumours could benefit from targeted treatment
Image: Infant high-grade glioma. Credit: David Ellison, St. Jude Children’s Research Hospital
Researchers at the ICR have discovered that brain cancer in babies is biologically distinct from other childhood brain tumours and could potentially be successfully treated with targeted drugs.
They looked at the genetic make-up of tumours from 241 infants diagnosed with glioma, an aggressive type of brain cancer.
In the study they found that 54 per cent of tumour samples had an entirely different genetic make-up than other forms of childhood brain tumours.
Over half of children with the distinct form of infant brain tumours, had specific molecular weaknesses – including ALK and NTRK gene fusions, which can be targeted with existing precision medicines.
A small number of children whose tumours were analysed in the study were successfully treated with ALK or NTRK targeting drugs.
Study leader Professor Chris Jones, Professor of Paediatric Brain Tumour Biology at the ICR, said:
“We showed that brain tumours in infants have particular genetic weaknesses that could be targeted with existing drugs – and clinical trials are planned to test the benefit of these precision medicines as a first-line treatment as soon as possible.”
The study was published in Cancer Discovery, and funded by charities including the CRIS Cancer Foundation, the Brain Tumour Charity, Children with Cancer UK, Great Ormond Street Hospital Children's Charity, and Cancer Research UK.
March 2019
AI could predict risk of lung cancer return
-945x532.jpg)
Image: Non-small cell lung cancer. Credit: Dr Ed. Uthman via Yale Rosen on Flickr, CC BY-SA 2.0
ICR scientists trained an artificial intelligence (AI) tool to determine which patients with lung cancer have a higher risk of their disease coming back after treatment.
Our researchers used the tool, which can spot the difference between immune cells and cancer cells, on samples from 100 patients with lung cancer and later validated in a dataset of 970 patients. They found that patients who had a higher number of areas of the tumour without immune cells – called ‘cold’ regions – were at a higher risk of relapse.
This tool could be used to give doctors more detailed information about the cellular make-up of tumours, informing tailored treatment strategies for individual patients.
Study leader Dr Yinyin Yuan, who leads the Computational Pathology and Integrative Genomics team at the ICR said:
“We’ve gained new insight into how lung cancers can cloak themselves to escape the attention of the immune system – and in doing so can continue to evolve and develop. Cancer’s ability to evolve and to come back after treatment is one of the biggest challenges facing cancer researchers and doctors today.”
The study was published in the journal Nature Medicine and was funded by Cancer Research UK.
February 2020
Trial shows targeted drug capivasertib may extend breast cancer survival
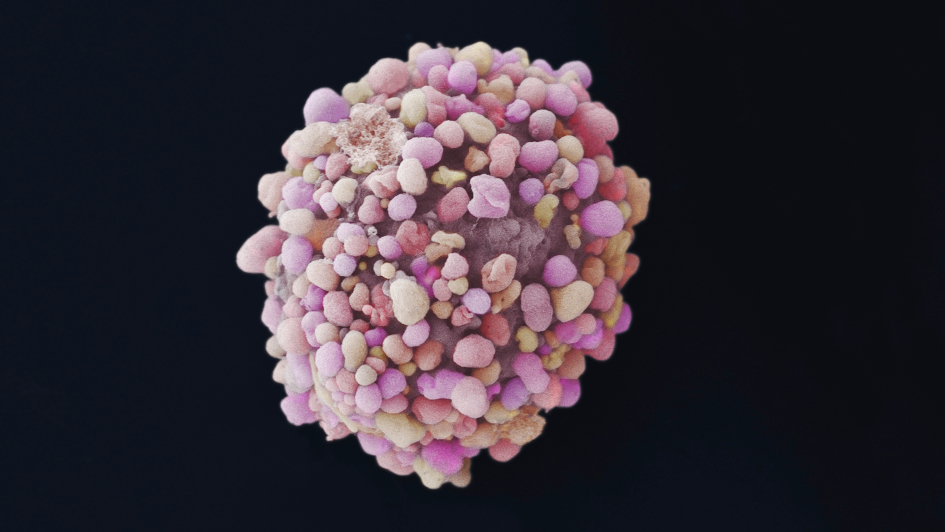
Image: Black and white electron microscope image of a breast cancer cell. Credit: Bruce Wetzel and Harry Schaefer, National Cancer Institute
A major international trial showed that women with a type of breast cancer that is particularly difficult to treat could benefit from a capivasertib – a cancer drug underpinned by discoveries made at the ICR.
Capivasertib was discovered following collaborative work between the ICR and partners including AstraZeneca, Astex and The Royal Marsden NHS Foundation Trust.
Women with ‘triple-negative’ breast cancer who received capivasertib alongside chemotherapy lived nearly seven months longer than those on chemotherapy alone – 19.1 months on average compared with 12.6.
Our researchers found that a specific subgroup of patients whose tumours had mutations in PIK3CA, AKT1 and PTEN genes may particularly benefit from capivasertib.
The trial, published in the Journal of Clinical Oncology, involved 140 patients in 42 academic centres across the world.
The drug is now being investigated for triple-negative breast cancer in a larger phase III trial.
Study co-leader Professor Nick Turner, Professor of Molecular Oncology at the ICR and Consultant in Medical Oncology at The Royal Marsden, said:
“Identifying a new targeted therapy treatment for triple negative breast cancers, with mutations in the PI3 kinase pathway, would be a major advance for breast cancer treatment. The results of this trial are highly promising, and we look forward to the results of the larger phase III trial.”
The research was supported by the National Institute for Health Research and Cancer Research UK funding to the Barts Experimental Cancer Medicine Centre. Additional funding was provided by AstraZeneca.
May 2020
Delays in cancer surgery could cost thousands of lives during COVID-19 pandemic
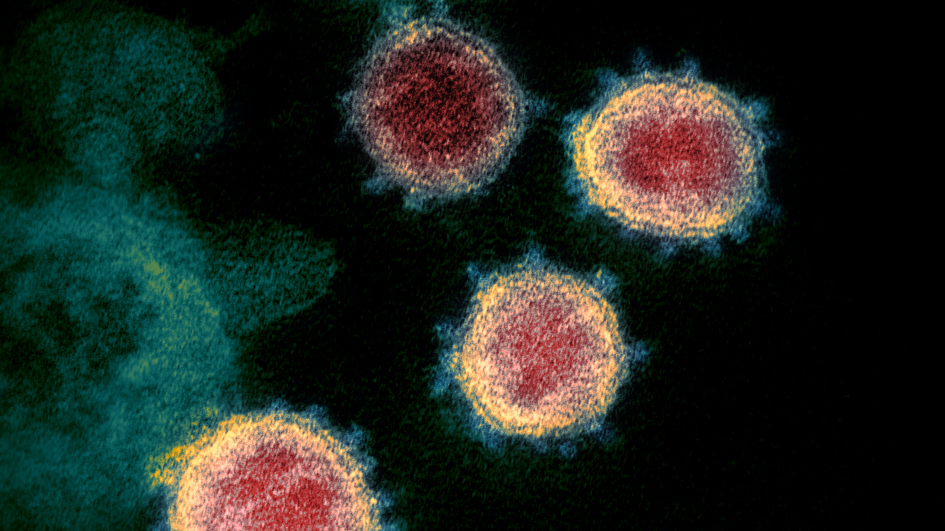
Image: Colorised transmission electron microscope image of SARS-CoV-2, the virus that causes COVID-19. Credit: National Institute of Allergy and Infectious Diseases, via Flickr. CC BY 2.0
A study led by ICR scientists found that delays to cancer surgery and other treatment during the COVID-19 pandemic could result in thousands of additional deaths in England.
Their research analysed existing Public Health England data on delays to cancer surgery on patients’ five-year survival rates to estimate the effect of three-month or six-month delays.
The modelling, which factored in the risk of hospital-acquired coronavirus infection, found that patients whose cancers will have progressed during the delay and who might otherwise have been effectively cured by surgery could now be at risk of their cancer coming back and shortening their lives.
The results of this study were published in Annals of Oncology.
Study leader Professor Clare Turnbull, Professor of Cancer Genomics, said:
“The COVID-19 crisis has put enormous pressure on the NHS at every stage of the cancer pathway, from diagnosis right across to surgery and other forms of treatment. Our study shows the impact that delay to cancer surgery will have on patients, with England, and the UK more widely, potentially set for many thousands of cancer deaths as a result of the pandemic.”
The new study was funded by the ICR itself, with support from Cancer Research UK.
July 2020
Lung cancer is different in smokers and non-smokers
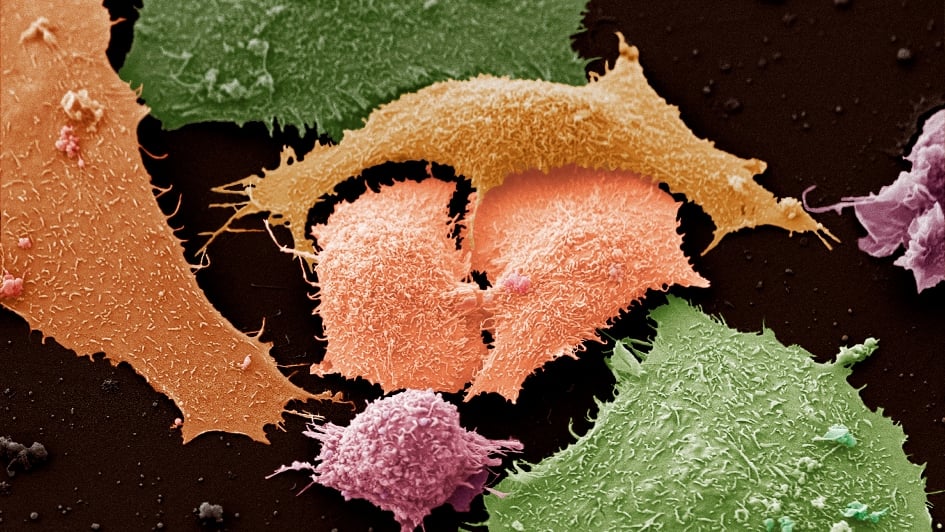
Image: Lung cancer cells. Credit: Anne Weston, CC BY-NC 4.0
Lung cancer is two distinct diseases in smokers and non-smokers that could respond differently to treatment.
ICR scientists studied a population in Taiwan with high rates of lung cancer among non-smokers – and found a range of changes to genes and proteins which varied depending on a patient’s age or sex.
Looking at the genetics and the associated protein signatures produced by cancer cells in the tumour samples, scientists found that some early-stage lung tumours in non-smokers were biologically similar to more advanced disease in smokers.
Picking out people with ‘late-like’ early-stage lung tumours could help guide treatment decisions, and patients could be monitored more closely for signs of their disease progressing.
Dr Jyoti Choudhary, Team Leader in Functional Proteomics at The Institute of Cancer Research, London, said:
“We carried out the most comprehensive study ever conducted into the biology of lung cancers in an East-Asian population with a high proportion of non-smokers, and found that their disease is molecularly diverse, and distinct from what we classically see in smokers.
“Some early-stage lung tumours had molecular features that are much more like that typically seen in later-stage disease – which could help us more accurately diagnose patients with aggressive disease, and guide treatment strategies.”
The research was published in the journal Cell, and was funded by Cancer Research UK and various institutions in Taiwan including the Ministry of Science and Technology.
April 2020
New molecule disrupts protein that drives lymphoma
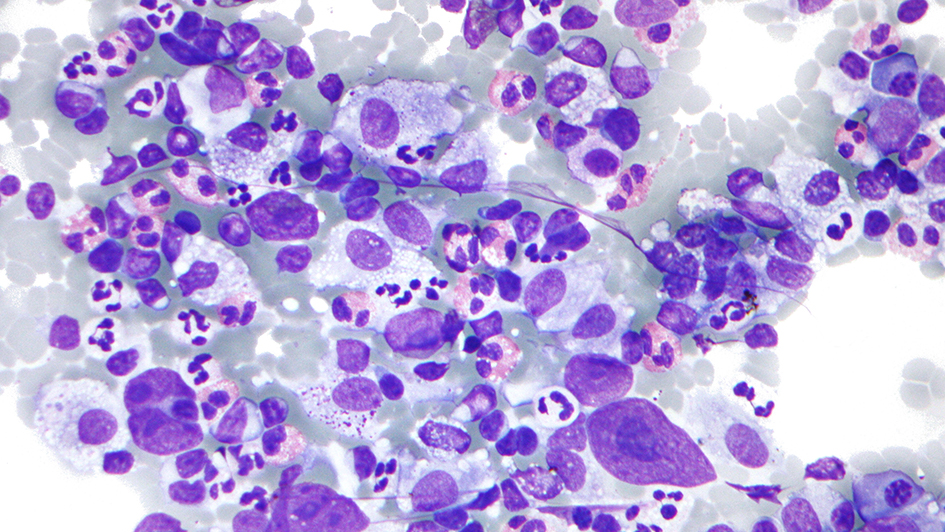
Image: A micrograph of Hodgkin lymphoma. Credit: Michael Bonert, CC BY-SA 3.0
Scientists at the ICR discovered and optimised a molecule that disrupts a protein involved in lymphomas, potentially identifying a new treatment target.
Our researchers were targeting a protein called BCL6 which plays a key role in antibody development in white blood cells – and when over-activated, can cause a range of lymphomas.
They investigated a series of molecules targeting the BCL6 protein, and found that one which they called CCT369260 was effective at disrupting it. When they tested the molecule in mice with diffuse large B-cell lymphoma, they found it caused a clear reduction in BCL6 levels within the tumours.
The team are now optimising the molecule’s structure to create further tools to confirm whether BCL6 could be a promising target for new treatments.
Dr Swen Hoelder, who led the study, said:
“This work suggests that future treatments to degrade BCL6 could be effective against haematological cancers. The molecule we identified can now be used to investigate the role of BCL6 degradation in treating lymphomas, and could lead to the creation of new drugs for the disease.”
The study was published in the Journal of Medicinal Chemistry and was funded by Cancer Research UK, the CRT Pioneer Fund and Sixth Element Capital.
June 2020
MRI scan used for heart disease could pick out aggressive cancers
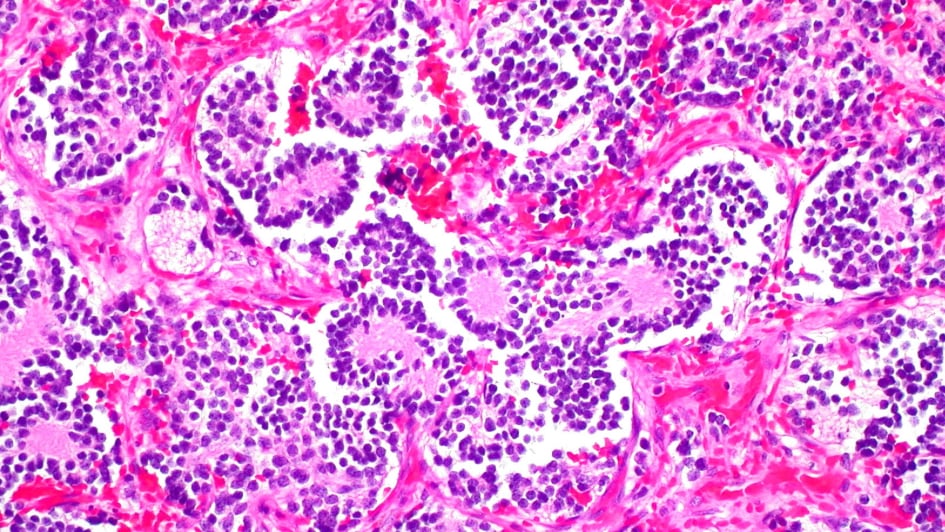
Image: Neuroblastoma of the adrenal gland. Credit: Ed Uthman. CC BY-NC 2.0
Researchers at the ICR found that a type of MRI scan used to assess damage to heart muscle tissue could also assess whether children’s cancers are especially aggressive and spot early signs that targeted treatments are working.
The MRI technique, known as T1-mapping, measures how water molecules interact at a microscopic level inside cells to understand the cellular make-up of tissue.
Researchers used the technique in mice with neuroblastoma to look at how they responded to two targeted drugs.
They found that when the drugs alisertib and vistusertib managed to stop the growth of tumours in mice, there was a decrease in T1 measures – reflecting the death of aggressive cancer cells.
Study leader Dr Yann Jamin, Children with Cancer UK Research Fellow at the ICR, said:
“Our findings show that T1 MRI scans have the potential to pick out children with aggressive cancer and give us early signs of whether a treatment is working. We’ve shown in mice that this technique can give us detailed insights into the biology of neuroblastoma tumours to help guide the use of precision medicine.”
The study, funded by Children with Cancer UK, Cancer Research UK and the Rosetrees Trust, was published in the journal Cancer Research.
April 2020
Driving cancer evolution into dead end could beat drug resistance
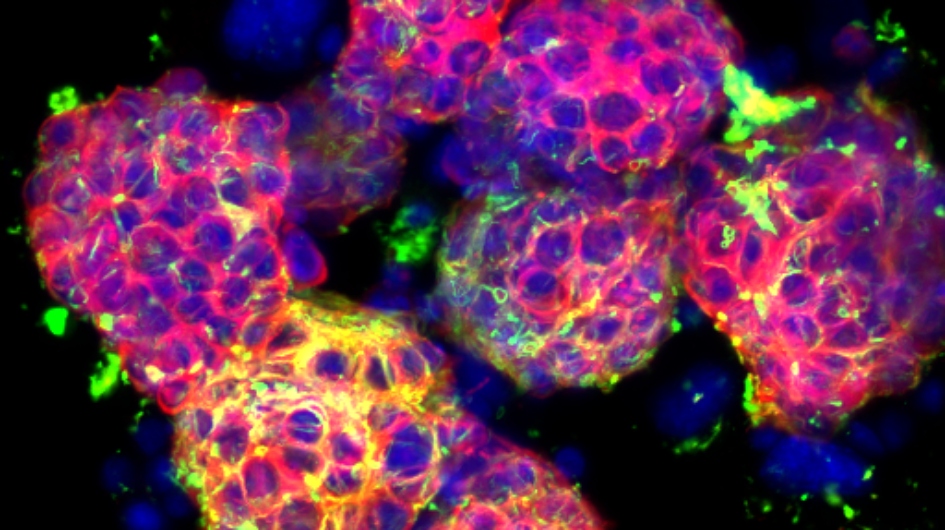
Image: Metastatic neuroblastoma (cropped). Credit: Orli Yogev, ICR
ICR researchers have found that it is possible to steer the evolution of cancer cells so that they develop vulnerabilities to treatment.
The findings could in future guide clinical decisions in treating cancer and help to overcome cancer’s ability to become drug resistant.
Cancer is difficult to treat because it can evolve and adapt to changes in its environment, including becoming resistant to therapy.
ICR scientists led by Professor Andrea Sottoriva studied lung cancer cells as they developed resistance to cancer drugs to explore these mechanisms.
Many of the cancer cells died in response to the drugs, but some drug-resistant cells survived and multiplied to become dominant.
They identified mutations that occurred early in tumour development which could offer clues about how tumours evolve to develop resistance, and could also be exploited to make cancer vulnerable.
Study leader Professor Andrea Sottoriva, Director of the Centre for Evolution and Cancer at the ICR, said:
“Understanding how tumours evolve in response to treatment is key to delivering better care for patients. Our research shows that we can manipulate cancer by steering its evolutionary development to leave it highly susceptible to treatment.”
By carefully selecting sequences of drugs to treat cancer, doctors could force cancer cells to adapt into evolutionary dead ends that can be targeted by treatments, leaving the disease no way to survive.
The research was published in the Journal Nature Communications, and was funded by Wellcome, Cancer Research UK and the National Institute of Health Research.
.tmb-propic-md.jpg?Culture=en&sfvrsn=d41885a5_9)
 .
.