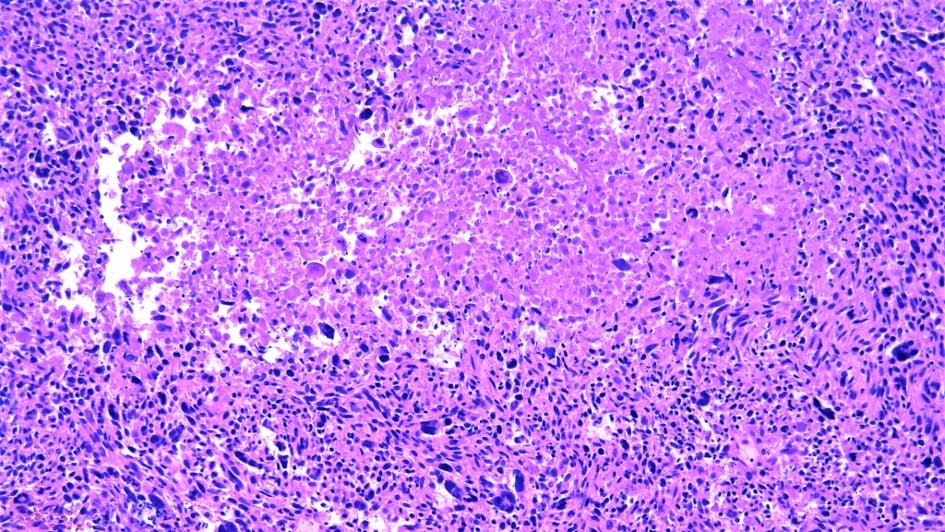
Image: Leiomyosarcoma. Credit: Wikimedia Commons.
A new study has answered the long-standing question of why improvements in survival outcomes for young people with cancerous soft tissue tumours have lagged behind those of their paediatric and older adult counterparts. By analysing the protein profiles across different types of soft tissue tumours, known as sarcomas, researchers have shown that there are distinct biological differences between these age groups.
Furthermore, the team has identified a potential biomarker that could help predict which adolescent and young adult (AYA) patients are likely to have aggressive forms of sarcoma that will spread to other areas of the body. In the future, this may help guide treatment decisions for people of this age, improving outcomes for patients predicted to need more intensive treatment and sparing those with less aggressive cancer from the side effects of overtreatment.
Researchers at The Institute of Cancer Research, London, led the study, which has been published in the journal Communications Medicine. The work was funded by various grants and charitable donations, with funders including the Sarah Burkeman Trust, Cancer Research UK, and the Children's Cancer and Leukaemia Group partnered with the Little Princess Trust.
Age affects outlook for people with soft tissue tumours
Soft tissue tumours develop in the connective and supporting tissues of the body, such as muscle and fat. They are relatively rare but can affect people of any age. Those that are malignant, or cancerous, are called sarcomas.
Despite the incidence of sarcoma being higher among the AYA age group than among older adults (representing 8 per cent of cancer diagnoses vs. just 1 per cent), improvements in survival rates in the AYA age group, defined as people aged 16 to 39 at the time of diagnosis, have not kept pace with those for patients belonging to other age groups.
The study authors note that there are multiple reasons for this disparity, including inadequate age-specific services and under-representation of people in the AYA age group in clinical trials. These factors mean that the current treatments are optimised for older adults – those aged 40 and older – and do not always work effectively for AYA patients.
Believing that biological differences between the age groups must play a part in these inconsistent outcomes, the researchers set out to retrospectively analyse the features of the sets of proteins expressed in patients of different ages. They used data from 309 people with soft tissue sarcomas or benign soft tissue tumours, known as desmoid tumours. Nine sarcoma types were represented across the cohort, including angiosarcoma, clear cell sarcoma and leiomyosarcoma.
Identifying a new way to predict cancer spread
The researchers identified a total of 8,148 proteins across all the patient samples and quantified 3,299 of them. They found that 32 of the proteins were more abundant in the AYA patients than in the older adults, while 35 were more abundant in the older adults. Once the researchers had adjusted the results to account for other variables, including tumour size, anatomical site and sarcoma subtype, five of these proteins remained significant.
Overall, the older patients had higher levels of a protein involved in regulating the cell cycle while the proteins that were more abundant in the AYA patients have a role in structural support and the function of mitochondria, which generate energy for cells.
These differences could affect how sarcomas respond to treatment, which will, in turn, influence people’s likelihood of survival.
In the next part of the study, the team wanted to determine whether there was a correlation between any biological factors and the survival rates of the participants. The analysis showed that high expression of specific subunits of the cellular machinery responsible for splicing – an important step in the process of protein synthesis – were associated with better metastasis-free survival (MFS). MFS is the time from the start of treatment until the cancer begins to spread.
This splicing “signature” could potentially be used by clinicians to identify the AYA patients most likely to need intensive treatment to prevent their cancer from spreading.
“This could lead to substantial improvements in survivorship”
First author Yuen Bun Tam, a PhD student in the Molecular and Systems Oncology Group at the ICR, said:
“The lack of therapies tailored to adolescent and young adult patients is a key barrier to improving survival rates in this age group, representing a significant unmet need. In this study, we not only characterised the biological differences between young people and older adult patients but also identified an age-specific signature that may serve as a risk stratification tool in the clinical setting.
“By demonstrating the importance of age-specific studies in the discovery of more tailored strategies, such as targeted agents, we hope that our findings will encourage future studies and clinical trials to include more adolescents and young adults. In the long term, this could lead to substantial improvements in survivorship and the management of late effects for this age group.”
Senior author Dr Paul Huang, Leader of the Molecular and Systems Oncology Group at the ICR, said:
“Although we predicted that there would be inherent biological differences between the tumours in the two patient groups, we were surprised to see that many of these findings were independent of other clinical factors that differed between the two age groups, including the type of sarcoma.
“We are planning further work to validate our findings in larger cohorts and to measure our splicing signature, with the hope of making it feasible to use as a clinical test. We will also work on better understanding the link between this splicing signature and the ability of the tumour to spread. This knowledge could aid the identification of new treatment options to manage soft tissue tumours in adolescents and young adults.”

 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
.jpg?sfvrsn=faf78b2_4) .
.
 .
.
 .
.
 .
.