Structural Biology of Cell Signalling
Dr Sebastian Guettler’s group is researching the ways in which certain enzymes, known as ADP-ribosyltransferases (ARTs), control cell function.
Professor Sebastian Guettler
Deputy Head of Division:
Structural Biology of Cell Signalling.jpg?sfvrsn=4c485212_1)
Professor Sebastian Guettler is Deputy Head of the Division of Structural Biology. He studies the precise molecular mechanisms of signalling processes central to cancer stem cell function, with a particular interest in Wnt/β-catenin signalling, telomere length homeostasis and their regulation by ADP-ribosylation. He has a long-standing interest in understanding tankyrase, a poly-ADP-ribosyltransferase with roles in both Wnt/β-catenin signalling and telomere maintenance.
Researchers in this group
 .
.
Email: [email protected]
Location: Chelsea
I completed my MChem at the University of York in Chemistry, Medicinal and Biological Chemistry, with a year in industry, where I worked at UCB Pharma, in the Medicinal Chemistry department. My Masters project was on a drug discovery project in peptide therapeutics. After this, I completed a 10-week internship in organic chemistry at AstraZeneca. I then worked as a Junior Medicinal Chemist for a year, at Autifony Therapeutics, working in CNS drug discovery. For my PhD, an MRC iCASE-funded project, I aim to develop small molecules targeting the telomeric Shelterin complex, jointly in Sebastian Guettler's and Swen Hoelder's groups at the ICR, with Merck as our industry partner.
 .
.
Phone: +44 20 3437 6624
Email: [email protected]
I graduated from the University of Southampton, with an integrated Master’s in Biochemistry (MSc). For my bachelor’s project, I investigated the effects of reactive oxygen species and vitamin C on S100A9 aggregation in Alzheimer’s disease. For my Master’s project, I investigated the functional importance of Snap29 for mitosis in Drosophila. For my PhD, I study the molecular mechanisms of telomere maintenance.
 .
.
I graduated from the University of Manchester, with a BSc in Biochemistry with industrial experience (IE). I spent my IE at Mayo Clinic in Jacksonville, Florida, investigating mitochondrial dysfunction in disease. In 2020, I joined the Guettler lab to study how tankyrase regulates Wnt/β-catenin signalling utilising genetic techniques. I completed my PhD in the end of 2024.
 .
.
Email: [email protected]
Location: Chelsea
Oviya studied Biotechnology during her undergraduate degree at SRM University, India. She then completed her PhD in Professor Sara Sandin's lab at Nanyang Technological University, Singapore. Oviya joined the ICR as a postdoc in 2019 and is currently studying the function of tankyrase at telomeres.
 .
.
I completed my BSc degree at the University of West Indies (UWI), in Kingston Jamaica. I then pursued an MSc degree at the University College London (UCL), where I characterised a novel cause of Silver-Russell Syndrome in the lab of Professor Gudrun Moore. Subsequently, I ventured off to Zürich, where I joined the lab of Professor Ulrike Kutay at the Institute of Biochemistry, ETH Zürich, as a PhD student. My PhD research identified mammalian inner nuclear membrane proteins that majorly contribute to the establishment and the maintenance of 3D genome architecture, and the resulting biological consequences when this organisation is perturbed. I joined the Guettler Lab as a Postdoc in October 2025, and my work involves investigating the molecular mechanisms of tankyrase, particularly in Wnt/β-catenin signalling and telomere length homeostasis.
 .
.
Katy completed her MChem degree at Durham University in 2023, where her final-year research project focussed on the application of peptidomimetics to generate novel therapeutics for Alzheimer's disease. At the ICR, she's working on a collaborative project with Professor Sebastian Guettler and Professor Swen Hoelder, developing novel scaffolding inhibitors of tankyrase.
 .
.
Dr Vasundara Srinivasan obtained her PhD in Biochemistry from the University of Georgia, USA in the lab of Prof B.C. Wang. She next joined the lab of the Nobel Laureate Prof Hartmut Michel at the Max Planck Institute of Biophysics, Germany to work on membrane proteins, G-protein coupled receptors (GPCR) and transporters, focussing on mitochondrial ABC transporters in collaboration with Prof Roland Lill. As a project leader at the VIB, Brussels, Belgium, she used nanobodies as chaperones for the crystallisation of GPCRs. She headed the crystallisation facility at the University of Marburg before joining the lab of Prof Christian Betzel at DESY, Hamburg to work on the structure-based drug discovery of proteases involved in the replication cycle of SARS-CoV-2. She joined the lab of Prof Sebastian Guettler at the ICR in January 2025.
Professor Sebastian Guettler's group have written 29 publications
Most recent new publication 4/2025
See all their publicationsResearch, projects and publications in this group
ADP-ribosylation is a post-translational modification carried out by ADP-ribosyltransferases (ARTs), which transfer ADP-ribose from NAD+ onto substrates. ADP-ribosylation controls many aspects of cell function, including DNA repair, cell division, telomere maintenance, chromatin dynamics, apoptosis and various signal transduction processes. Given their roles in DNA repair, telomere homeostasis and cancer-relevant signalling pathways, several ARTs are being explored as potential cancer therapy targets.
In humans, the family of intracellular ARTs encompasses 17 members with similar catalytic domains but greatly diverse non-catalytic accessory domains. Different catalytically active ARTs can either transfer a single unit of ADP-ribose or attach ADP-ribose processively, thereby constructing poly(ADP-ribose) (PAR) chains, which can be of varying length and structure. Enzymes in the latter group are known as poly(ADP-ribose)polymerases (PARPs). Compared to other types of post-translational modification, such as phosphorylation, PARylation remains understudied.
We take a particular interest in the PARP enzyme tankyrase, which fulfils a wide range of biological functions, many of which are relevant to cancer. The human genome encodes two highly similar tankyrase paralogues, TNKS and TNKS2. Both share a C-terminal catalytic PARP domain, a set of five N-terminal ankyrin repeat clusters (ARCs) responsible for substrate recruitment, and a polymerising sterile alpha motif (SAM) domain in between.
Our previous structure-function work has revealed the mechanisms of substrate recognition and polymerisation by tankyrase and shown that tankyrase can act as a scaffolding protein, independently of its catalytic function. We now aim to use both X-ray crystallography and cryo-electron microscopy to understand how tankyrase’s various domains act together. Moreover, we work with chemists to develop novel approaches to modulate tankyrase function.
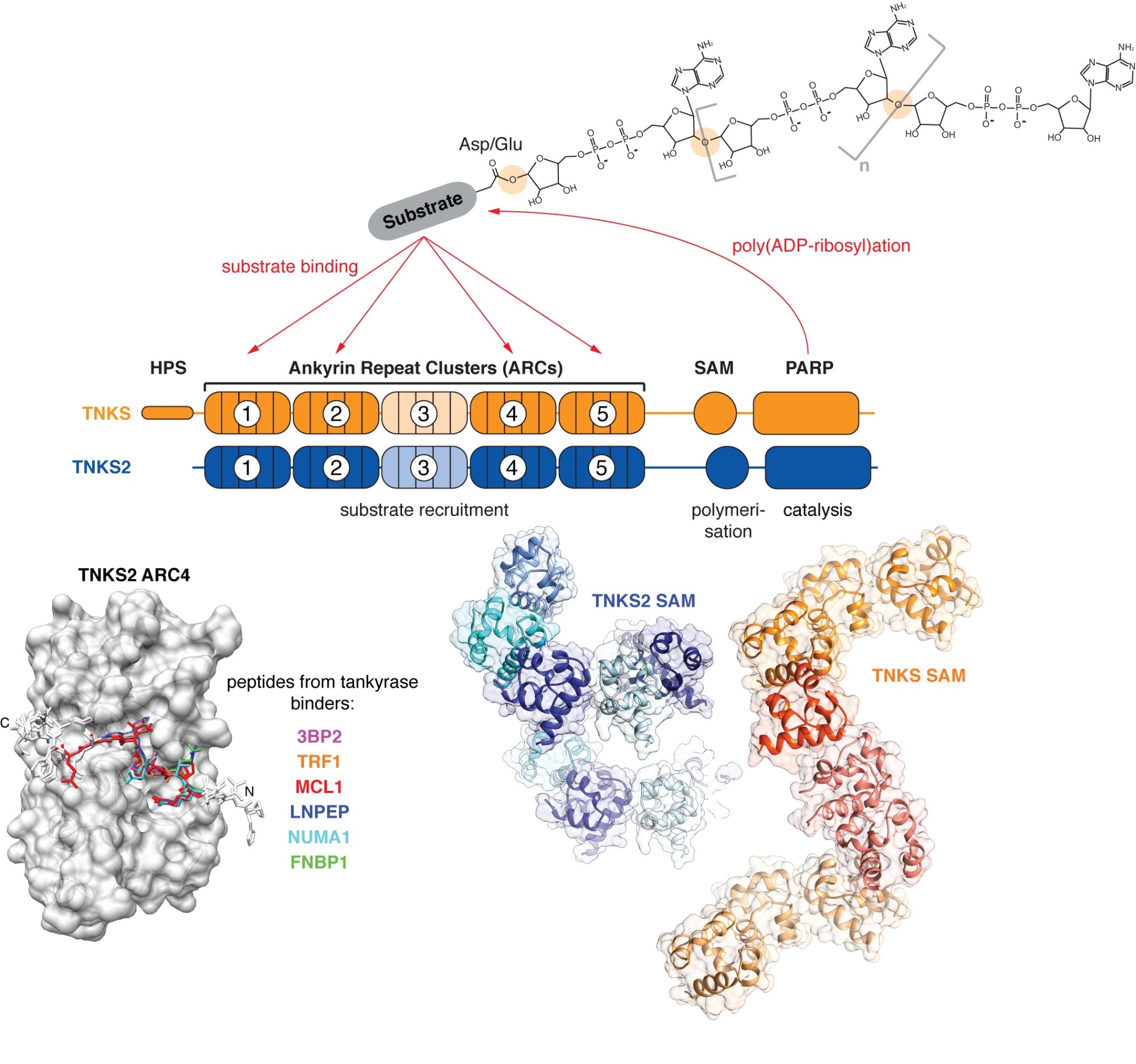
Tankyrase (TNKS, TNKS2) uses its ankyrin repeat clusters (ARCs) to recruit binding partners, many of which are also PARylated by tankyrase’s PARP domain. ARCs recognise degenerate peptide motifs found in many proteins. Our earlier work (Guettler et al., 2011) has revealed the substrate recognition mechanism and explained how the rare human disease Cherubism is caused. The sterile alpha motif (SAM) domain enables tankyrase polymerisation. We have revealed the mechanism of tankyrase polymerisation and shown that both ARCs and the SAM domain polymer fulfil essential scaffolding roles and are required for efficient substrate modification (Mariotti et al., 2016). (Images modified from Guettler et al., 2011; Mariotti et al., 2016 and Pollock et al., 2017)
Molecular mechanisms of Wnt/beta-catenin signalling, telomere maintenance and their regulation by poly(ADP-ribosyl)ation
In a series of projects, we take a reductionist approach to study how large macromolecular complexes coordinate Wnt/beta-catenin signalling and telomere length homeostasis and how they are controlled by tankyrase-dependent poly(ADP-ribosyl)ation. We combine biochemical assays with cryo-electron microscopy and X-ray crystallography to uncover the detailed mechanisms governing the functions of these complexes and their regulation.
Besides uncovering fundamental mechanisms underlying stem and cancer cell function, we endeavour to understand the molecular basis of disease mutations and open up new opportunities for pharmacological intervention.
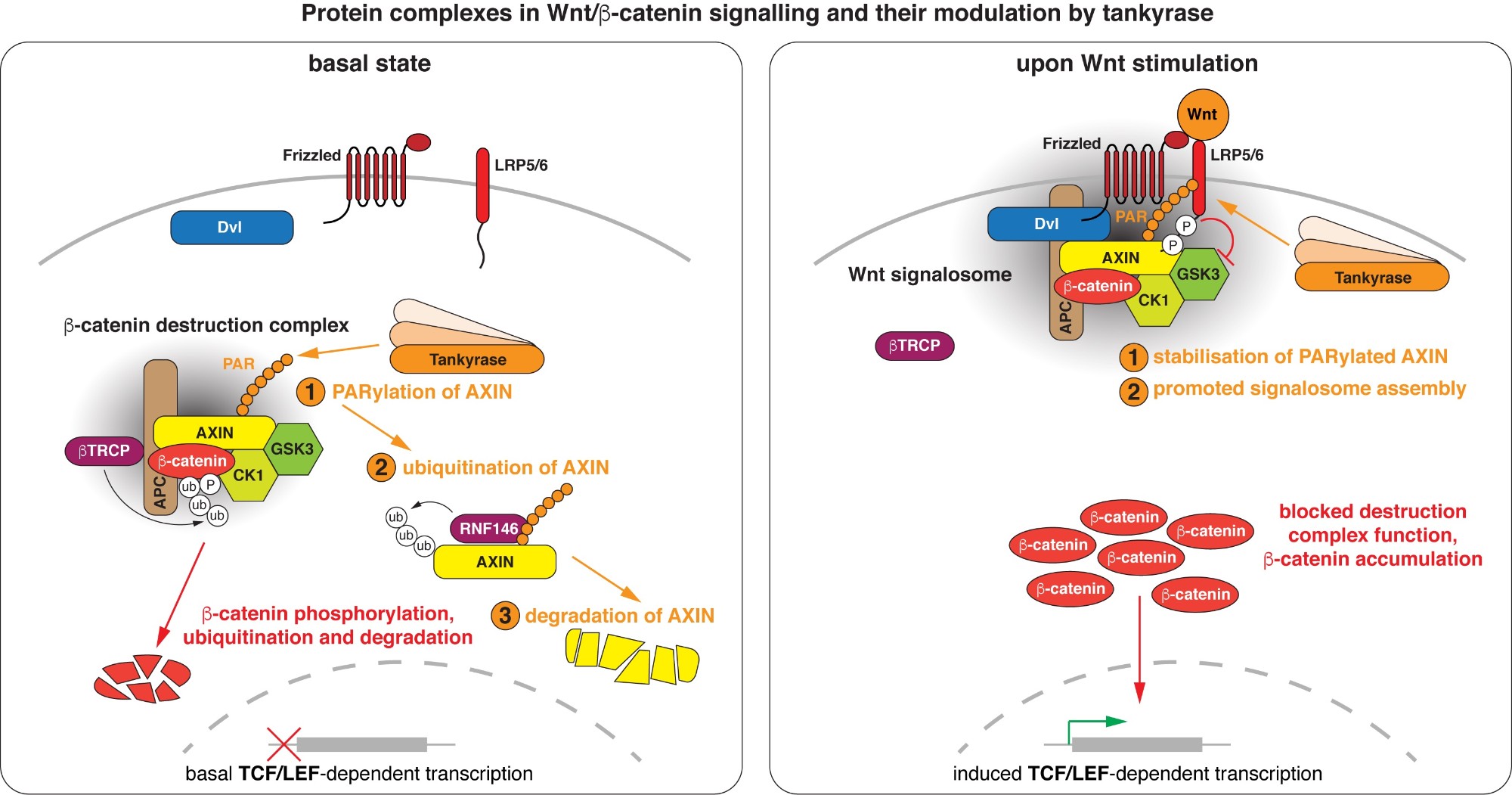
Wnt/beta-catenin signalling revolves around controlling the levels of the transcriptional co-activator beta-catenin. A multi-protein beta-catenin destruction complex captures cytoplasmic beta-catenin and limits its abundance by initiating its phosphorylation- and ubiquitination-dependent degradation. Notably, destruction complex function is impaired in the vast majority of colorectal cancer cases. Wnt stimulation remodels the destruction complex into a membrane-localised “Wnt signalosome” incapable of destabilising beta-catenin. Tankyrase controls the receptiveness of cells to incoming Wnt signals by PARylating AXIN, thereby destabilising the destruction complex or promoting Wnt signalosome formation. (Images modified from Mariotti et al., 2017)
A small number of key signalling pathways collaborate to confer stem-cell properties to cells, and the Wnt/beta-catenin pathway is a prototypic example for such a pathway. Wnt/beta-catenin signalling plays important roles in embryonic development and adult organ homeostasis. It is dysregulated in a number of different cancer types, most prominently in colorectal cancers, the vast majority of which bear mutations in components of the pathway.
At the same time, stem and most cancer cells rely on active telomerase to prevent erosion of their telomeres and maintain their unlimited replicative potential. Recent findings show that Wnt/beta-catenin signalling and telomere homeostasis are closely intertwined at multiple levels and form an integrated self-renewal programme, relevant to normal tissue regeneration, ageing and cancer.
The poly(ADP-ribose)polymerase (PARP) tankyrase both promotes Wnt/beta-catenin signalling and is essential for normal telomere extension in humans, thereby providing an important link between both processes.
Our overarching goal is to understand the precise molecular mechanisms that underlie Wnt/beta-catenin signalling, telomere maintenance and their control by poly(ADP-ribosyl)ation. We have a long-standing interest in deciphering the structural basis and molecular mechanisms of tankyrase function.
We employ biochemistry, structural biology and cell biology to study the molecular mechanisms of Wnt/beta-catenin signalling and telomere homeostasis, with a particular focus on how poly(ADP-ribosyl)ation (PARylation) controls both processes. Besides understanding fundamental mechanisms of cell function, we aim to uncover novel potential therapeutic avenues in cancer.
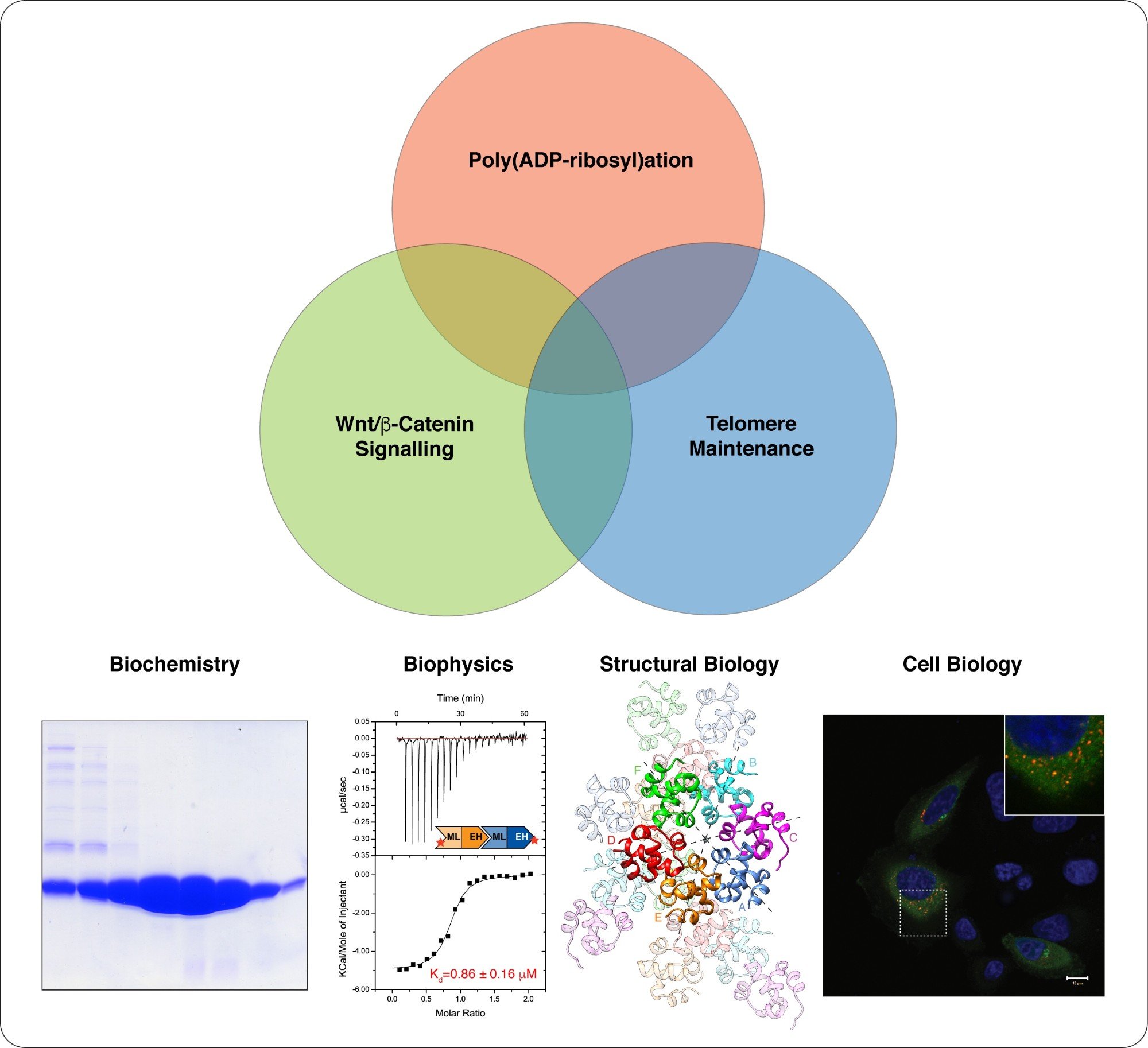
We take a multidisciplinary approach to study Wnt/beta-catenin signalling, telomere maintenance and their regulation by poly(ADP-ribosyl)ation. Structural biology is at the centre of our work. (Images modified from Mariotti et al., 2016)
Vacancies at the ICR
Working at the ICR
Research Group Leader, ICR Clinical Trials and Statistics Unit (ICR-CTSU)
Department/Directorate Information: Division of Clinical Studies - Clinical Trials and Statistics Unit (ICR-CTSU) The ICR-CTSU is a Cancer Research UK-funded, internationally recognised methodologist led clinical trials unit, providing cancer-focused clinical trial research expertise. We lead pioneering, efficient, high-quality, and impactful trials across the phases. Our expertise ranges from experimental medicine early phase studies exploring biological efficacy to trials which may deliver widespread change to routine practice, underpinned by applied methodology to drive forward clinical trial innovation. See our clinical trials Role Summary The Group Leader will lead a component of ICR-CTSU’s portfolio of clinical trials research. The post holder will join an existing faculty and seek to further develop and grow the portfolio in line with ICR-CTSU’s overall strategy; taking responsibility for a number of ongoing trials as well as the development of new trials. There will be the opportunity to develop and grow a team including the potential for a Postdoctoral Training Fellow and/ or a Trial Manager. We seek an experienced statistician / biostatistician with a strong research interest in clinical trials methodology and a passion for direct involvement in the oversight and leadership of academic clinical trials. The successful candidate will work closely with the Director of ICR-CTSU to further enhance the Unit’s internationally recognised strength in clinical trial design, conduct and analysis. The post holder will be expected to make a substantial independent intellectual contribution to clinical trials projects and be proactive in leading and contributing to broad initiatives that enhance the overall effectiveness of ICR-CTSU. The appointee will contribute to the overall scientific life of the ICR including the newly established ICR/Royal Marsden Hospital’s Centre for Trials and Population Data Science, by providing mentorship to more junior colleagues and acting as an academic leader. We seek an individual who will work closely and collaboratively with other faculty/Group Leaders at the ICR and with international/national key opinion leaders to extend the breadth and depth of ICR-CTSU’s biologically rich clinical trials portfolio. In partnership with clinical opinion leaders, this individual will generate research funds to conduct and deliver clinical trials research at the international forefront. Presentation at national and international conferences, production of top-quality research outputs and substantial professional contribution to wider clinical trial network bodies are expected. Enthusiasm for team-based science in a collaborative interdisciplinary environment is essential. The appointment will be based on track record and the ability and willingness to engage in team science. The successful appointee will have access to ICR’s successful PhD training programme and core facilities. Key Requirements Higher degree (MSc or PhD) in medical statistics/biostatistics or an allied field (e.g. public health, epidemiology, data science) with relevant work experience Significant experience as a clinical trials, medical statistician or bio-statistician within the academic or commercial sector; a blend of both would be highly desirable A desire to apply existing and novel statistical methods to the requirements of a diverse range of statistical problems A broad understanding of cancer research Ability to lead a Clinical Trials Unit based research group As part of your online application, you will be required to upload your full CV which will pre-populate your application form, you will also be asked to attach the following documents and failure to do so will mean your application cannot be considered on this occasion: Lists of major publications, achievements, research grants and distinctions. A PDF of a maximum of five key publications, or other research outputs (e.g. patents) that best demonstrate previous productivity or a single document giving hyperlinks to these outputs. You must also complete the personal statement section of the application form in the format of a cover letter including the names and contact details of three academic referees Joining as a Group Leader, you will be given outstanding support to help you to continue to develop in your career. Along with a start-up package of funding, you will also have access to resources to establish your group, including support for recruiting key group members, such as PhD students and postdoctoral researchers. We encourage all applicants to access the job pack attached for more detailed information regarding this role. For an informal discussion regarding the role, please contact Professor Emma Hall ([email protected])
Digital Manager – Website Lead
About the Role We’re looking for a talented Digital Manager to take ownership of the ICR’s main website and lead its evolution. You will manage, optimise and grow our website following its major redevelopment on the latest version of the Sitefinity CMS. This is a pivotal, highly collaborative role at the heart of a busy directorate that tells the ICR’s story and leads our fundraising. Key Responsibilities As our Website Lead, you will: Oversee daily management, development and optimisation of the ICR’s main website — including content, SEO/AEO and technical improvements Work closely with internal partners to develop new pages, sections and features Lead a programme of ongoing content review and user training across the organisation Produce regular website analytics reports and deliver insight-driven recommendations Ensure consistent branding, accessibility and outstanding user experience Manage a Digital Communications Officer (job share, 1.2 FTE) and help recruit and line-manage a new Website Developer Plan and prioritise technical projects with our Digital Services (IT) team About You This is a fantastic opportunity for someone who combines technical understanding with creativity, editorial judgement and a passion for delivering exceptional digital experiences. You’ll bring: Strong experience managing and publishing content within a CMS (Sitefinity experience is a bonus) A solid understanding of HTML and confidence working with developers and IT colleagues Experience overseeing the day-to-day running of a large website Skills in analysing website performance using tools like GA4, Google Tag Manager or Matomo Excellent organisational ability and the skills to manage multiple concurrent projects Strong written and verbal communication skills Experience managing or mentoring others (highly desirable) A proactive, collaborative approach to working across teams Optional but advantageous: experience with Adobe Creative Cloud tools, editorial content review, and training non-technical users What We Offer A supportive and collaborative working environment. Opportunities for professional development and career progression. Competitive salary and pension About the Development and Communications directorate The Development and Communications directorate tells the ICR’s story and focuses on income generation. The ICR is world-renowned for its outstanding cancer research – and it deserves communication to match. We believe that communicating effectively about the ICR's work can help us build on our successes – attracting donors and supporters, the best staff and students, commercial partners and collaborators. We encourage all applicants to access the job pack attached for more detailed information regarding this role. For an informal discussion regarding the role, please contact Eleanor Howard, Head of Strategic Marketing via email on [email protected].

Employee Story
Dr Federico Tidu is a Postdoctoral Training Fellow in Dr Christian Zierhut’s Cancer Biology – Genome Stability and Innate Immunity Team. He and his team are based in our Chester Beatty Laboratories building in Chelsea, Central London.
"Working in Chelsea is great and there’s a very good community here."
Industrial partnership opportunities with this group
Opportunity: Biomarker for CDK4/6 and/or aromatase inhibitor response in breast cancers
Commissioner: Dr Maggie Cheang
Recent discoveries from this group



