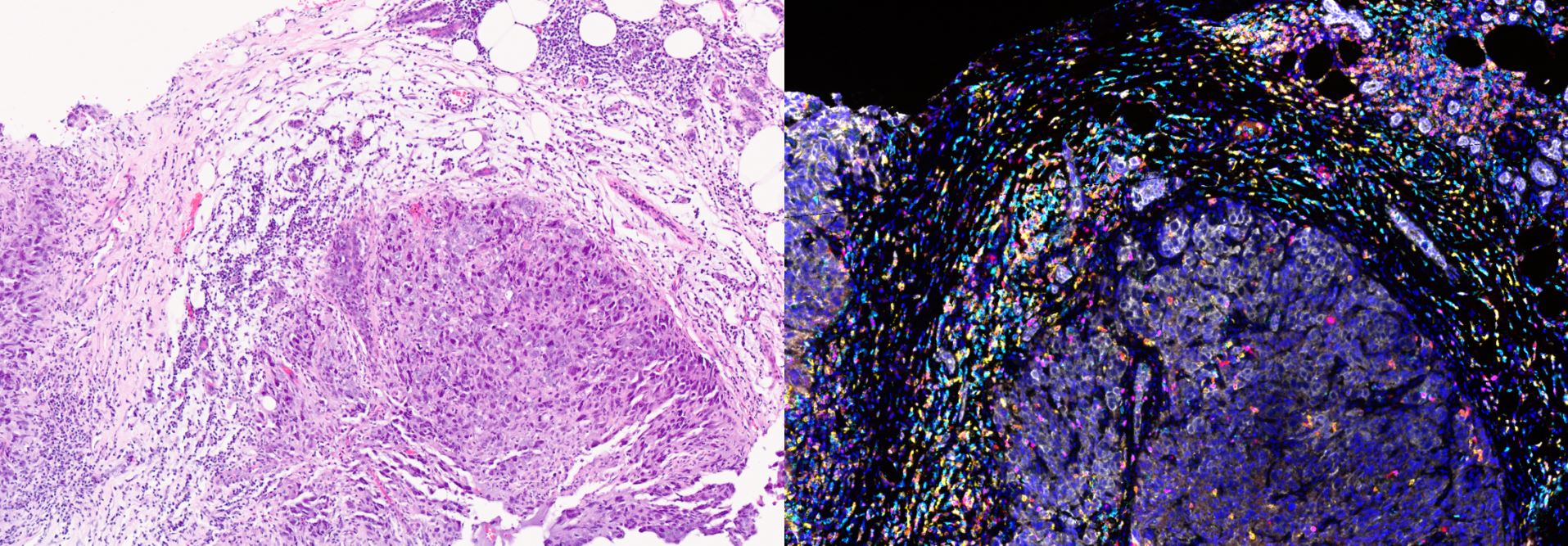
Clinical Pharmacology and Trials Group (including Drug Metabolism and Pharmacokinetics Group)
Professor Udai Banerji's group conducts early investigations of new anticancer agents developed in the Centre for Cancer Drug Discovery.
Research, projects and publications in this group
Our group studies preclinical and clinical pharmacology, and is responsible for the early clinical trials, of new anti-cancer agents developed in the Centre for Cancer Drug Discovery.
Professor Udai Banerji
Group Leader:
Clinical Pharmacodynamics Biomarker Group, Clinical Pharmacology & Trials, Clinical Pharmacology Adaptive Therapy Group
Professor Udai Banerji champions multidisciplinary working at the interface between early phase clinical trials, drug discovery and translational research related to drug resistance. He is Deputy Director of the Drug Development Unit at The ICR and Royal Marsden, and as a key member of the Centre for Cancer Drug Discovery, also heads the Clinical Pharmacodynamics Biomarker Group and the Clinical Pharmacology-Adaptive Therapy Group.
Researchers in this group
Professor Udai Banerji's group have written 138 publications
Most recent new publication 1/2025
See all their publicationsThe Clinical Pharmacology and Trials Group is responsible for the study of the preclinical and clinical pharmacology, and for the early clinical trials, of new anti-cancer agents developed in the Centre for Cancer Drug Discovery.
Such investigations may include the study of mechanisms of action and resistance, toxicology, pharmacokinetics, early dose-finding studies and the development of pharmacodynamic biomarkers for the measurement of drug action in tumour and surrogate tissues. These may be molecular assays or functional imaging studies. The emphasis is increasingly on hypothesis-testing clinical trials of agents acting on new molecular targets including cell signalling, cell survival, the cell cycle control machinery, chromatin modulation and angiogenesis.
The Drug Metabolism and Pharmacokinetics (DMPK) Group (led by Dr Florence Raynaud), within the Clinical Pharmacology and Trials Group, is a multidisciplinary group studying the pharmacokinetics of anti-cancer drugs in clinical trials. The group helps to characterise and prioritise the best drugs to enter the clinic: once in clinical trials, the group conducts and analyses pharmacokinetic data crucial to make go/no-go decisions, as well as decisions on optimal doses and schedules of novel anti-cancer drugs.
The Clinical Pharmacodynamic Biomarker Group works with drug discovery groups to transfer assays used in drug discovery to platforms that can be used to assay human tissue sampled during clinical trials.
Aims
- To conduct first-in-human hypothesis-testing phase I studies of novel anti-cancer drugs
- The clinical development of new discoveries within the Centre for Cancer Drug Discovery
- To discover, develop and apply new pharmacodynamic markers
- Define the clinical context for novel targets and develop combination strategies for novel agents discovered at the ICR.
- Develop non-invasive imaging techniques for evaluation of pharmacodynamic effects of anticancer agents in tumours.
 .
.