Molecular Cell Biology Group
Professor Clare Isacke’s Group investigates the environments surrounding breast cancer cells to understand the molecular basis of the disease’s progression.
Research, projects and publications in this group
Our group collaborates closely with other groups within the Breast Cancer Now Research Centre, the Division of Breast Cancer Research and colleagues at the Royal Marsden NHS Foundation Trust.
Professor Clare Isacke
Group Leader:
Molecular Cell Biology
Professor Clare Isacke aims to identify the processes by which tumour cells recruit non-cancerous cells during metastasis. She has held several senior positions at the ICR, including acting as Interim Head of the Division of Breast Cancer Research, and is currently Academic Dean.
Researchers in this group
Professor Clare Isacke's group have written 141 publications
Most recent new publication 5/2024
See all their publicationsCancer as a disease can be regarded as having three broad stages:
- Uncontrolled growth of primary tumour
- Invasion into adjacent tissues
- Metastasis, in which tumour cells escape from the primary site and re-establish growth at distant, secondary locations
In breast cancer, as with other cancers of epithelial origin, it is well recognised that these proliferative, invasive and metastatic events do not result solely from rogue cancer cells acquiring additional capabilities and behaving abnormally within 'normal' surroundings.
Rather, all three stages rely on the ability of the tumour cells to recruit and activate neighbouring non-tumour (stromal) cells and, additionally, to respond to the signals that these activated stromal cells produce. Further, there is now increasing evidence that these activated stromal cells can modulate the response of tumour cells to both targeted therapies and cytotoxic chemotherapy.
Consequently, in our laboratory, a focus is placed on tumour cells in the context of their cellular and non-cellular environments, particularly the metastatic microenvironment. Our goal is to identify pathways and processes that can be targeted for the prevention or suppression of secondary disease or which are responsible for treatment-resistant tumour progression.
In Professor Isacke's group, a focus is placed on tumour cells in the context of their cellular and non-cellular environments and to identify the pathways and processes that can be targeted for the prevention or suppression of metastatic disease or which are responsible for treatment-resistant tumour progression.
The work involves close collaborations with other groups within the Breast Cancer Now Research Centre, the Division of Breast Cancer Research and colleagues at the Royal Marsden NHS Foundation Trust.
The group's current work can be divided into the following themes:
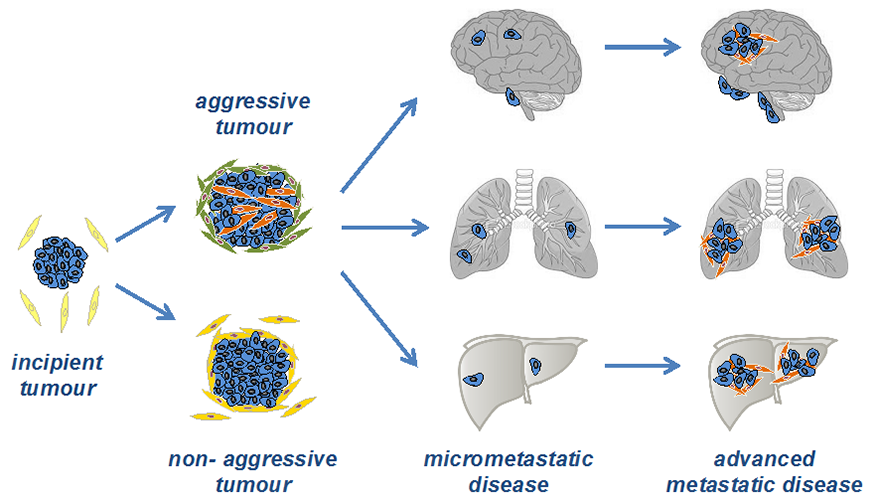
1) Mechanism and function of cancer-associated fibroblast (CAF) and pericyte activation during breast cancer progression
There is now extensive functional evidence implicating a role for cancer-associated fibroblasts (CAFs) in tumour progression, either by their ability to deposit and remodel the extracellular matrix, by the secretion of pro-tumourigenic factors or by modulation of the immune compartment.
Our laboratory has been addressing the mechanisms that underpin fibroblast heterogeneity in breast cancers. First, by gene expression profiling of aggressive and non-aggressive tumours, we have identified Wnt7a as a key tumour cell-secreted factor that promotes both fibroblast recruitment and activation in three-dimensional in-vitro assays and in vivo studies. Functionally, high Wnt7a expression in tumour cells promotes metastasis in vivo and, in human breast cancers, correlates with a desmoplastic, poor-prognosis stroma accompanied by high TGFbeta pathway activation in fibroblasts and reduced patient survival (Avgustinova et al., 2016).
Currently, we are exploring the recruitment of CAF subsets during the establishment of a favourable metastatic microenvironment and the role of CAFs in driving an immune suppressive microenvironment in the tumour that underpins the clinical observation that breast cancers are often refractory to immunotherapy.
Pericytes are a mesenchymal cell population that function to stabilise quiescent resting vessels; however, their role in both developmental and tumour angiogenesis is less well understood. Endosialin (also known as CD248) is a large transmembrane glycoprotein that was reported originally to be one of the most highly upregulated genes in the tumour vasculature compared to normal vasculature.
Our laboratory was the first to demonstrate that endosialin is not expressed on endothelial cells but on the activated pericytes (MacFadyen et al., 2005; MacFadyen et al. 2007; Simonavicius 2010) where it binds to a vascular basement membrane components and regulators of vascular branching (Simonavicius et al., 2012).
In collaboration with Hellmut Augustin's laboratory (DKFZ Heidelberg) we have demonstrated that endosialin-positive pericytes actively facilitate metastatic spread by promoting tumour cell intravasation and subsequent dissemination (Viski et al., 2016).
Currently, we are exploring the utility of targeting fibroblast/pericyte activation receptors upregulated in the tumour stroma focusing initially on endosialin (see above) and the endocytic recycling receptor Endo180 (MRC2, uPARAP).
Endo180 is predominantly expressed by stromal fibroblasts, functioning as a pro-migratory, novel collagen uptake receptor (Wienke et al., 2003; Sturge et al., 2006; Huijbers et al., 2010). More recently, we demonstrated that genetic deletion of Endo180 severely impairs colonisation of tumour cells at the metastatic sites.
2) Identifying novel modulators of metastasis
The dissemination of primary tumour cells to secondary sites cannot be modelled in vitro. Consequently, we have taken in vivo approaches, particularly in vivo shRNA screens in syngeneic models, to identify novel modulators of breast cancer metastasis.
These approaches have resulted in (a) the identification of ST6GalNAc2 as a novel metastasis suppressor that functions to limit tumour cell extravasation into metastatic sites (Murugaesu et al., 2014); (b) the demonstration that JNK1 signalling is upregulated by chemotherapy treatment, that JNK inhibitors limit chemotherapy cytotoxicity and that monitoring JNK activity has utility as an early biomarker of chemotherapy response (Ashenden et al., 2017); and (c) the identification of the aldo-keto reductase AKR1B10 as a metastasis promoter that functions to protect tumour cells from oxidative stress-induced damage at metastatic sites, thereby allowing a switch from glycolysis to an energy efficient, fatty acid oxidation-fuelled metabolism (van Weverwijk et al. in revision).
We have also taken two approaches to identifying drivers of breast cancer metastasis to the central nervous system. First, based on gene expression profiling of 4T1 sublines isolated from primary tumours, lungs and brains, we have identified Id2 as an enhancer of breast cancer colonisation of the brain parenchyma.We showed that Id2 expression in disseminated breast cancer cells is elevated due to stimulation from astrocyte-derived BMP7, providing a survival advantage to tumour cells in the absence of matrix attachment (Kijewska et al. in revision).
Second, in collaboration with Andrew Tutt, we have initiated a project to identify molecular drivers of breast cancer leptomeningeal metastasis (BCLM). Leptomeningeal metastasis remains a devastating feature of breast cancer, with median survival of only 4 months, no widely-accepted standard of care for treatment, limited access to clinical trials and no molecular biomarkers for monitoring disease progression or treatment response.
However both cell-free tumour DNA (ctDNA) and circulating tumour cells can be isolated from cerebrospinal fluid (CSF). We have collected and sequenced (whole genome and exome) CSF ctDNA, plasma ctDNA, primary tumour DNA, non-brain metastatic lesion DNA and germline DNA, revealing unique aberrations in CSF ctDNA not shared by other metastatic sites.
In addition, we have optimised protocols for the isolation of CSF-derived tumour cells and subsequent generation and tagging of patient derived organoids (PDOs) for in vitro and in vivo validation studies.
3) Development of new models to study metastatic relapse
The development of metastatic disease can occur rapidly after initial diagnosis or many years after removal of primary tumour and systemic treatment, referred to as metastatic dormancy. The metastatic microenvironment plays a key role in controlling if and when disseminated tumour cells switch to a proliferative phase giving rise to clinically detectable metastatic disease.
Early metastatic relapse is particularly associated with chemo-refractory triple-negative breast cancers whereas late (>5 years) relapse is associated with ER+ breast cancers. Laboratory studies on metastatic relapse are hampered by the lack of robust in vivo models, particularly syngeneic models and models of ER+ breast cancer.
To overcome these limitations we have (a) derived and characterised metastatic sublines from the non-metastatic mouse D2A1 mammary tumour cell line (Jungwirth et al., 2018), (b) identified and characterised a series of syngeneic and PDX models to investigate the role of the metastatic microenvironment in ER+ breast cancer relapse, (c) developed ex vivo and in vitro models with which to study the role of stromal cell activation in promoting release of tumour cells from dormancy.
Further information on Professor Isacke's research projects can be found on the Breast Cancer Now Breast Cancer Research Centre website.
 .
.
.jpg?sfvrsn=b8be1e0b_2)