Endocrine Control Mechanisms Group
Professor Cathrin Brisken's group aims to understand how hormones drive breast cancer development to better prevent the disease and tumour recurrence.
Research, projects and publications in this group
We have developed powerful in vivo and ex vivo approaches to study how hormones impinge on hormone-sensitive breast cancer cells in physiologically relevant conditions.
Professor Cathrin Brisken
Group Leader:
Endocrine Control Mechanisms
Professor Cathrin Brisken leads the Endocrine Control Mechanisms Group. Her research aims to understand how recurrent exposures to hormones contribute to breast cancer in order to better prevent and treat the disease.
Researchers in this group
 .
.
Dr Beatrice Howard is a Staff Scientist in the Endocrine Control Mechanisms Group at the ICR. She specialises in embryonic breast development and its relationship to breast cancer.
Professor Cathrin Brisken's group have written 124 publications
Most recent new publication 6/2025
See all their publicationsA woman’s risk to get breast cancer is affected by her reproductive history and exposures to endogenous and exogenous estrogen receptor (ER), progesterone receptor (PgR), and androgen receptor (AR) ligands are linked to the pathogenesis of ER+ breast cancer which represent more than 70% of all cases. Our understanding of the cellular and molecular mechanisms by which the ovarian hormones impinge on breast carcinogenesis have been hampered by the lack of adequate models to study this. Our laboratory has overcome this hurdle by demonstrating that the correct tissue microenvironment is critical for human breast cells to maintain their hormone receptor expression and signaling. By xenografting cells to the milk ducts of immunocompromised mice we obtain take rates for previously non graftable cell lines and patient-derived cells of >90%. The mouse intraductal xenograft (MIND) models critical stages of disease progression, including metastasis, in clinically-relevant endocrine conditions and reflect intra and inter-tumor heterogeneity. We are exploiting mice with humanized breast epithelia to determine the mechanisms underlying sex hormone action in human ER+ breast carcinogenesis.
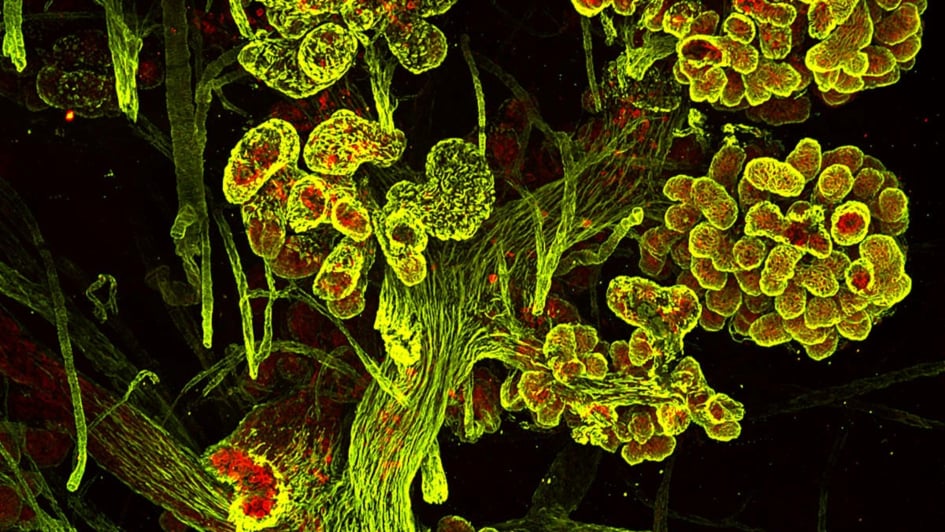
3D reconstruction of the human breast. In red cytokeratin 7 and in green smooth muscle antigen. Credit: Patrick Aouad
Targeting the progesterone receptor (PR) in ER+ breast cancer
Although the major ovarian hormones, estrogens and progesterone, both drive postnatal breast development and are linked to carcinogenesis, endocrine therapies currently target the ER only with selective ER modulators (SERM), selective ER degraders (SERD), or estrogen-lowering aromatase inhibitors. These drugs have and improved survival dramatically but 30% of patients ultimately recur with resistant disease. We explore targeting the PR as an alternative strategy to prevent and/or treat endocrine resistant disease.
The PR is co-expressed with the ER, both in the normal breast epithelium and in ER+ breast cancers and the two pathways are intertwined at multiple levels. Using genetic and pharmacologic tools we aim to determine the role of the PR in tumor growth and metastasis. The use of patient-derived xenografts allows us to dissect what determines a tumor’s response to hormone receptor signaling.
Lobular Carcinoma of the Breast
Fifteen precent of breast cancers are of a special histopathological type called lobular carcinoma. Lobular carcinomas are typically slow growing and hormone sensitive but they have a high risk of late recurrence, a unique metastatic pattern and pathognomonic loss of E-cadherin function. Progress in our understanding of this particular type of breast cancer has been hampered by the lack of models to study it.
We have shown that lobular carcinoma cells from cell lines and from patient tumors when grafted directly to the milk ducts of immunocompromised female mice, grow, invade, and metastasize as they do in patients. Molecular analyses of patient derived xenografts (PDXs) revealed that the ILC cells actively secrete and modulate extracellular matrix important to their survival (Sflomos G et al, EMBO Mol Med. 2021).
We are exploring whether a clinically approved inhibitor of the secreted enzymes of the LOX family that are critical for crosslinking collagen fibers and other ECM components can be exploited therapeutically and whether we can identify endpoints to enable clinical trials.
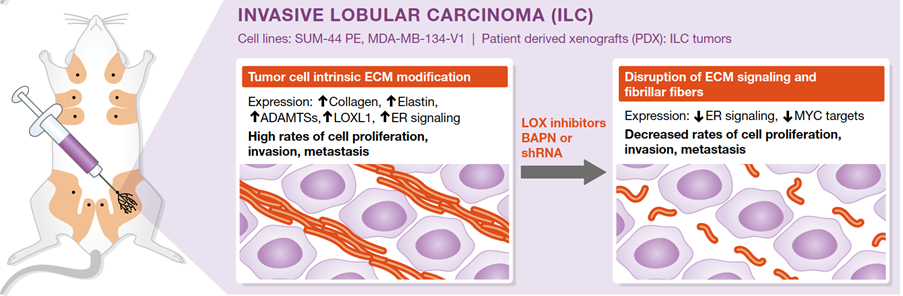
The intraductal (MIND) system reveals lobular carcinoma-specific biology and vulnerabilities. Adapted from Kozma et al.
New Patient-Derived In Vivo Models
In close collaboration with clinical partners we establish patient-derived MIND models of ER+ breast cancers to reflect the clinical complexity of the disease both in terms of intra and inter patient heterogeneity and patient genetic ancestry.
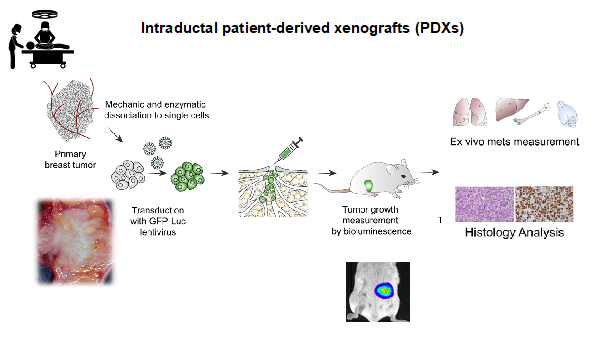
Workflow of the MIND patient-derived model establishment. Courtesy Valentina Scabia.
Resources
- Professor Brisken's laboratory at EPFL
- Preclinical Models course
- ITN PhD training network in cancer prevention (coordinated by Professor Brisken)
References
Sflomos, G., Battista, L., Aouad, P., DeMartino, F., Scabia, V., Ayyanan, A., Ifticene-Treboux, A, RLS, Bucher, P., Ambrosini, G., Fiche, M.,, Brisken, C. Intraductal Xenografts Model Lobular Carcinoma of the Breast and Reveal Matrix Remodeling by LOXL1 as a Targetable Hallmark. EMBO Mol Med. 2021.
Previewed: Kozma KJ, Done SJ, Egan SE. The tumor cell-derived matrix of lobular breast cancer: a new vulnerability. EMBO Mol Med. (2021)
Koch, C, .Kuske, A., Josse, S.A., Yigit, G., Sflomos, G., Thaler, S., Smit, D.J., Werner, S., Borgmann, K., Gärtner, S., Mohammadi, P.M., Battista, L., Cayrefourcq ,L., Altmüller, J., Salinas-Riester, G., Raithatha, K., Zibat, A., Goy, Y., Ott, L., Bartkowiak, K., Tan, Z. T., Speicher, M.R., Müller, V., Jücker, M., Thiery, J.P., Brisken, C.*, Riethdorf, S.*, Alix-Panabières, C*., Pantel, K. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity *equal contribution. EMBO Mol Med. (2021) Sep 7;12(9):e11908.
Cartaxo, AL., Estrada, M., Domenici, G., Roque, R., Silva, F., Gualda, EJ., Loza-Alvarez, P., Sflomos, G., and Brisken, C. , Alves, PM., André, S., Brito, C. A novel culture method that sustains ERα signaling in human breast cancer tissue microstructures. J Exp Clin Cancer Res 2020 Aug 17;39(1):161.
Siersbæk, R., Scabia, V., Chernukhin, I., Papachristou, E., Broome, R., Green, A., Joosten, S., Kumar, S., Alvarez, R., Nagarajan, S., Glont, S., Omarjee S., Aitken, S., Kishore,K., Rakha, E., D’Santos, C., Zwart, W., Russell, A., Brisken, C, Carroll, J. IL6/STAT3 co-opts ER regulatory elements to drive metastasis in breast cancer. Cancer Cell. 2020 Jul 6:S1535-6108(20)30311-1.
Laszlo, C. F., De Martino, F., Montoya, J. P., Shamseddin, M., Beguin, A., Nellen, R., Bruce, S., Moniatte, M., Henry, H., and Brisken, C. A high resolution LC-MS targeted method for the concomitant analysis of 11 contraceptive progestins and 4 steroids. J Pharm Biomed Anal. 2019 Oct 25;175:112756.
Vincent, GP., Nacht, AS, Ferrari, R., Zaurin, R., Scabia, V., Carbonell, J., Le Dily, F., Quilez, J., Leopoldi, A., Brisken, C, Beato, M. C/EBPα mediates the growth inhibitory effect of progestins on breast cancer cells. EMBO J. 2019 Sep 16;38(18):e101426.
Fiche, M., Scabia, V., Aouad, P., Battista, L., Treboux, A., Stravodimou, A., Zaman, K., RLS, Ayyanan, A., Sflomos, G., and Brisken, C. Intraductal patient derived xenografts of ER+ breast cancer recapitulate the histopathological spectrum and metastatic potential of human lesions. J Pathol. 2019 Mar;247(3):287-292.
Sflomos, G., Dormoy, V., T. Metsalu, T., Jeitziner, R., Battista, L., Scabia, V., Raffoul, W., Delaloye, J.-F., Treboux, A., Vilo,J., Fiche, M., Ayyanan, A., Brisken C. (2016) A robust preclinical model for ERα positive breast cancer points to the mammary epithelial microenvironment as critical determinant of luminal phenotype and hormone response. Cancer Cell, 2016 Mar 14; 29(3):407-422.
Previewed: Haricharan S, Lei J, Ellis M. Mammary Ductal Environment Is Necessary for Faithful Maintenance of Estrogen Signaling in ER(+) Breast Cancer. Cancer Cell. 2016 14;29(3):249-50.
Featured in: Research Watch: Bledsoe, K. Milk Duct Engraftment Allows Preclinical Modeling of ER+ Breast Cancer Milk Duct Engraftment Allows Preclinical Modeling of ER+ Breast Cancer. Cancer Discovery. 2016
Current vacancies in this group
Recent discoveries from this group
Senior Scientific Officer - Hormone-Dependent Cancer Models & Multimodal Data Integration
We are looking to recruit a Senior Scientific Officer to manage the Endocrine Control Mechanisms laboratory team and the dataset generated, lead by Professor Cathrin Brisken. The laboratory investigates endocrine mechanisms in breast development and breast carcinogenesis with advanced patient-derived intraductal xenograft (MIND/PDX) models the lab has pioneered. We seek a highly motivated and experienced Senior Scientific Officer for operational management and technical expertise across collaborative research projects. The successful candidate will support and coordinate a multidisciplinary team, ensure high-quality execution of experimental work, oversee data management processes and drive development of sophisticated in-vivo and molecular assays. Interviews will likely take place on Wednesday 11th / Thursday 12th February 2026. Endocrine Control Mechanisms Group What we offer A dynamic and supportive research environment Access to state-of-the-art facilities and professional development opportunities Collaboration with leading researchers in the field Competitive salary and pension We encourage all applicants to access the job pack attached for more detailed information regarding this role. For an informal discussion regarding the role, please contact Professor Brisken on [email protected]
 .
.
.png?sfvrsn=110bfd2c_1)