MA32
A phase III randomised trial of metformin versus placebo on recurrence and survival in early stage breast cancer
Disease site: Breast cancer
Treatment Modality:
Status: Closed
Trial details
Metformin, an oral agent commonly used worldwide to treat diabetes, has been associated with reduced overall cancer risk and mortality in diabetic patients and may be associated with enhanced responses to neoadjuvant systemic therapy in diabetic women with breast cancer.
Recent molecular studies have demonstrated that metformin results in initiation of an AMPK-dependent energy stress response which can adversely affect survival of breast cancer cell lines and inhibition of PI3K/Akt/mTOR signalling leading to reduced proliferation of breast cancer cell lines.
Recent neoadjuvant “window of opportunity” trials have confirmed a direct effect of metformin on breast cancers in vivo. Thus, metformin may exert anti-tumour effects through both insulin dependent and insulin-independent mechanisms in women with a broad range of insulin levels commonly seen in newly diagnosed breast cancer.
The National Cancer Institute of Canada Clinical Trials Group's MA 32 protocol, in which UK centres will participate, aims to evaluate the activity of metformin versus placebo on recurrence and survival in early stage breast cancer.
Aims & Objectives
To compare invasive disease free survival between breast cancer patients treated with metformin versus placebo in addition to standard adjuvant therapy.
Methods
Approximately 4,480 patients (200 in the UK) with invasive breast cancer who have been diagnosed and have undergone definitive surgery within the previous 12 months will be randomised in a 1:1 ratio to receive metformin (850 mg po bid for 5 years) or matching placebo in addition to standard adjuvant therapy.
How the results will be used
As metformin is a widely available treatment with an established toxicity profile (of mild gastrointestinal upset when initially taking the drug), it is likely that, if positive, the results of this large, multi-national trial will inform the decision to implement metformin as an adjuvant treatment for early breast cancer.
UK Chief Investigator: Professor Alastair Thompson, The University of Dundee
ICR-CTSU Scientific Lead: Professor Judith Bliss
Trial management contact: Natalie Atkins - Trial Manager, [email protected]
ISRCTN: TBC
Sponsor: National Cancer Institute of Canada Clinical Trials Group
Funding: Cancer Research UK (CRUK/11/034), NCIC
A summary of MA32 trial results for patients is available on the CRUK website.
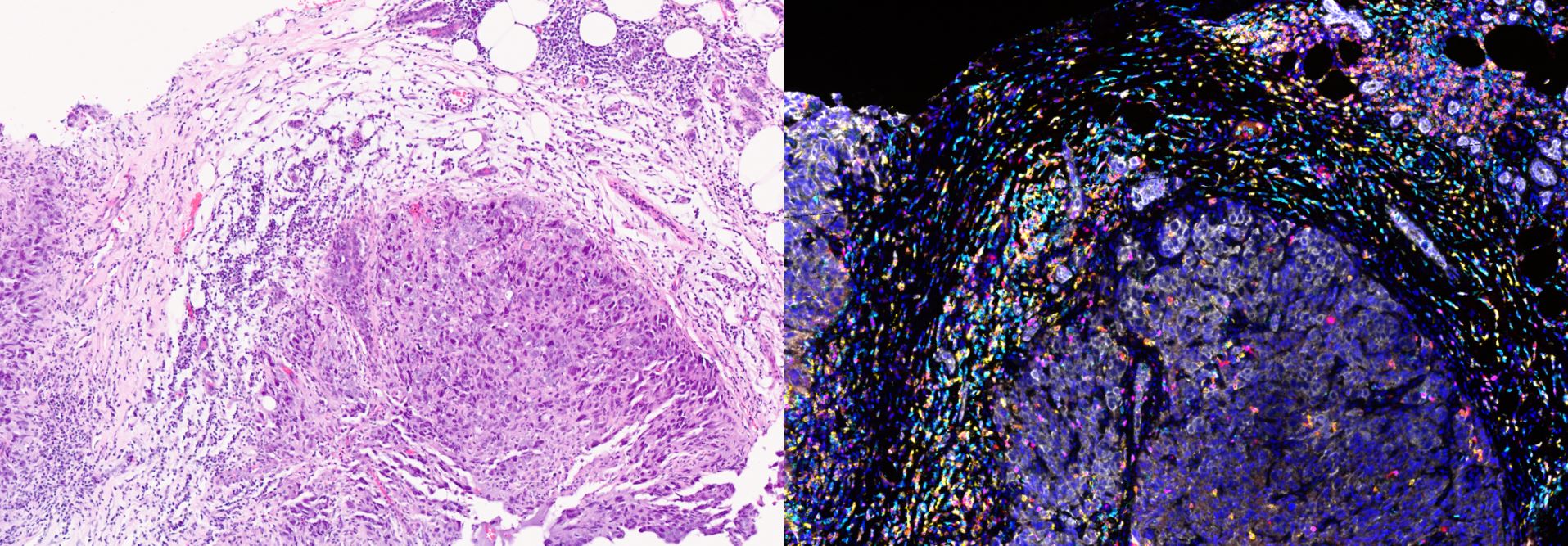
Neoadjuvant radiotherapy provides unique insights into breast tumour immune microenvironment