DEVA
A multicentre randomised trial of sequential epirubicin and docetaxel versus epirubicin in node positive postmenopausal breast cancer.
Disease site: Breast cancer
Treatment Modality:
Status: Closed
Trial details
The DEVA trial was run by the International Collaborative Cancer Group (ICCG) at Imperial College London in collaboration with ICR-CTSU.
Chief Investigator: Professor R. C. Coombes, Imperial College London
ICR-CTSU Scientific Lead: Professor Judith Bliss
ISRCTN: 89772270
Sponsor: International Collaborative Cancer Group (ICCG)
Funding: Pfizer, Sanofi-Aventis
Further information
Further information on the DEVA trial may be found on the following sites:
Patient friendly summary of the DEVA trial results at CancerHelp UK
Publications and presentations
Coombes RC, Bliss JM, Espie M, et al. Randomized, phase III trial of sequential epirubicin and docetaxel versus epirubicin alone in postmenopausal patients with node-positive breast cancer. J. Clin. Oncol. 2011;29(24):3247-54
Coombes RC, Bliss JM, Espie M, et al. DEVA: Randomized trial of sequential epirubicin and docetaxel versus epirubicin alone in node-positive postmenopausal early breast cancer (EBC) patients. J. Clin. Oncol. 2010;28(15_suppl):536
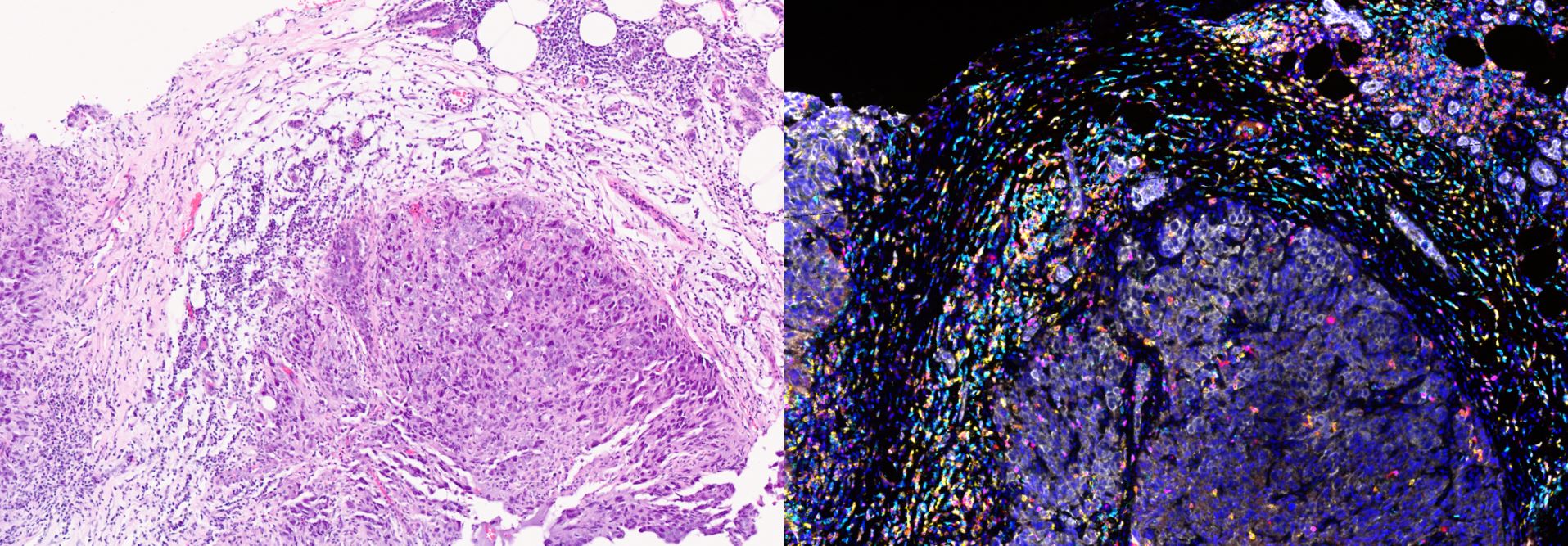
Neoadjuvant radiotherapy provides unique insights into breast tumour immune microenvironment