ABC
UK Co-ordinating Committee on Cancer Research randomised trial of adjuvant endocrine therapy and chemotherapy in women with early breast cancer.
Disease site: Breast cancer
Treatment Modality:
Status: Closed
Trial details
ABC is a randomised trial to evaluate whether adding chemotherapy and/or ovarian suppression increases the benefits of prolonged (5 years) tamoxifen for women with early breast cancer.
The trial launched in 1993 and closed to recruitment in September 2000 after recruiting 3,854 patients. All participants received tamoxifen. Pre- and peri-menopausal women received ovarian suppression or chemotherapy according to clinical judgement. Post-menopausal women were randomised to receive either adjuvant chemotherapy or no chemotherapy.
ABC is co-ordinated by the ICR-CTSU in collaboration with the Information Services Division Scotland Cancer Clinical Trials Team; the Cancer Research UK Clinical Trials Unit, University of Birmingham; the Clinical Trials Research Unit, University of Leeds; and the Ministry of Health Clinical Trials and Epidemiology Research Unit, Singapore. International collaboration with centres in the Middle East, Asia, Malta and New Zealand was an important aspect of this trial, with approximately one-third of patients recruited from international centres.
Follow up continues to date and associated quality of life and economic evaluation studies continue to mature.
Further information
Chief Investigator: Professor John Yarnold, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust
ICR-CTSU Scientific Lead: Professor Judith Bliss
Trial management contact: [email protected]
ISRCTN: 31514446
Sponsor: The Institute of Cancer Research
Funding: Cancer Research UK (CRUK/00/003) and Medical Research Council
Publications and presentations
The Adjuvant Breast Cancer Trials Collaborative Group. Polychemotherapy for early breast cancer: results from the international Adjuvant Breast Cancer chemotherapy randomized trial. J Natl Cancer Inst. 2007;99(7):506-15.
The Adjuvant Breast Cancer Trials Collaborative Group. Ovarian ablation or suppression in premenopausal early breast cancer: results from the international Adjuvant Breast Cancer ovarian ablation or suppression randomized trial. J Natl Cancer Inst. 2007;99(7):516-25.
Harnett AN, Bliss JM, Johnson L, Lawrence D, Raina V, Robinson A; ABC Trial Management Group. Thromboembolic events (TEE) in UK NCRI Adjuvant Breast Cancer (ABC) international trial – retrospective and prospective audits. J Clin Oncol. ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004:22(14S) Suppl July 15:666.
Yarnold JR, Bliss JM, Earl H, George D, Lawrence D, Mortazavi SH, Perren TJ, Raina V, Spooner D; ABC Trial Management Group. Ovarian ablation (OA) in pre-menopausal women with early breast cancer prescribed 5 years tamoxifen (T) or T plus chemotherapy (CT) – results from the UK NCRI Adjuvant Breast Cancer (ABC) international trial of 2,144 patients. J Clin Oncol. ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004:22(14S) Suppl July 15:535.
Barrett-Lee PJ, Bliss JM, Brunt AM, Dehshiri K, Harnett AN, Johnson L, Lawrence D, Robinson A, Weerasekera K; ABC Trial Management Group. Polychemotherapy (CT) in pre- and post-menopausal women with early breast cancer prescribed 5 years tamoxifen (T) – results from the UK NCRI Adjuvant Breast Cancer (ABC) international trial in 1991 patients. J Clin Oncol. ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004:22(14S) Suppl July 15:656.
Brunt AM, Bliss JM, Benghiat A, Dawson C, Dewar J, Harnett AN, Hopwood P, Lawrence D, Trask C; ABC Trial Management Group. The impact on quality of life of adding chemotherapy (CT) or ovarian suppression (OS) to adjuvant tamoxifen (TAM): outcomes from the UK NCRI Adjuvant Breast Cancer (ABC) trial. J Clin Oncol. ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004:22(14S) Suppl July 15:8015.
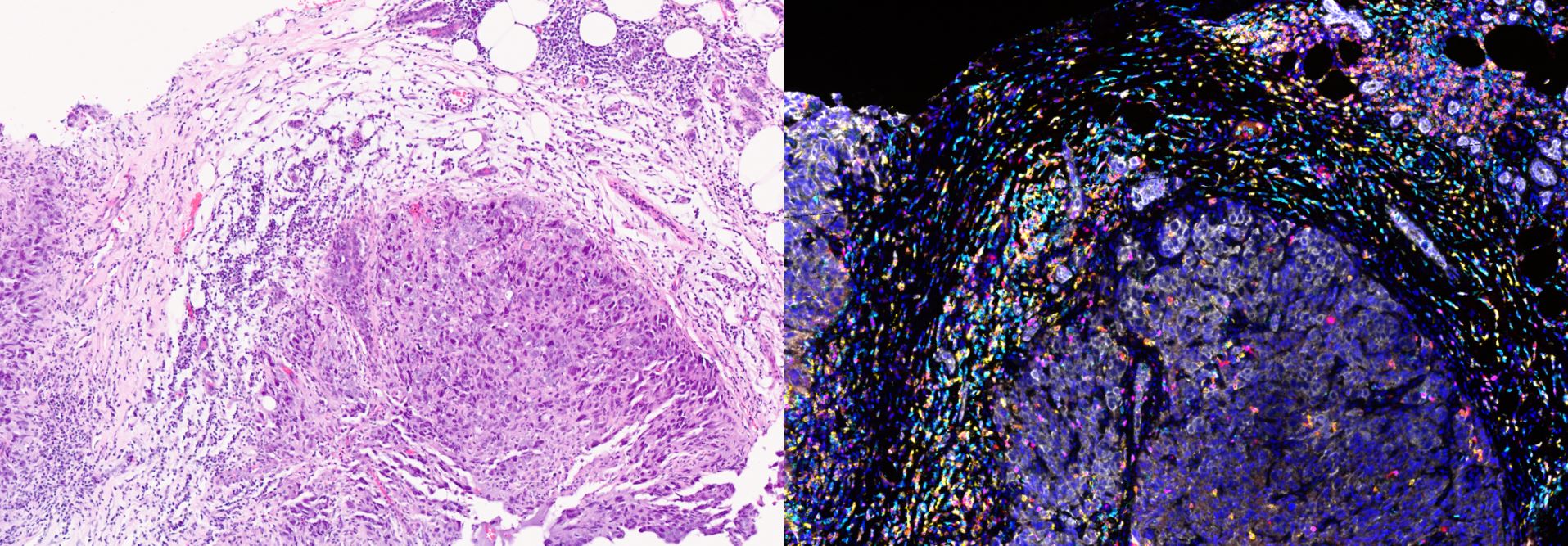
Neoadjuvant radiotherapy provides unique insights into breast tumour immune microenvironment