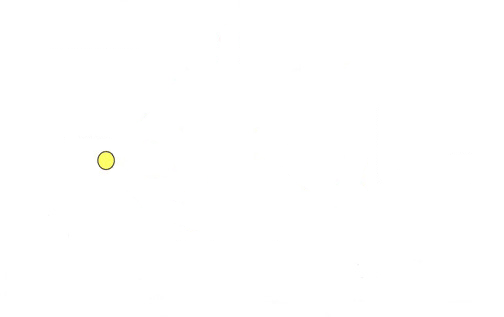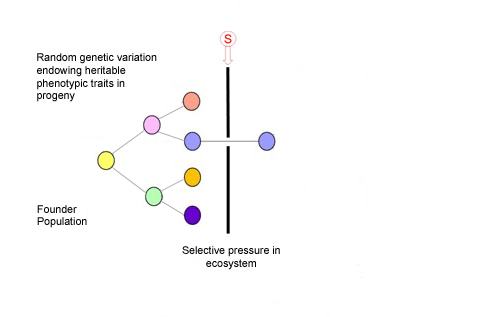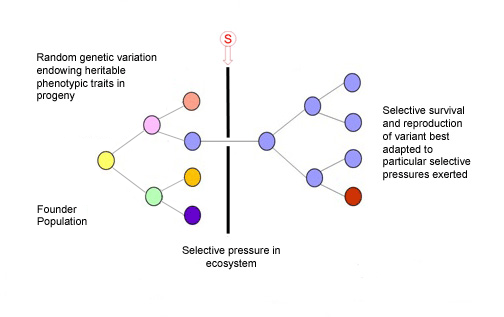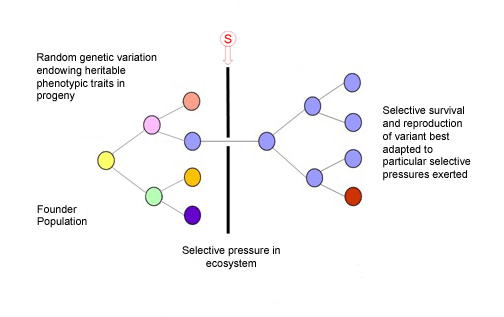
Charles Darwin had it right, despite knowing nothing of genetics or the basis of inheritable variation. This illustration shows us how evolution and speciation works for microbes, fungi, plants, animals, and, essentially, it is also how cancer works.
But genetic diversification and natural adaptability of somatic cells certainly isn’t all bad news. It has also been harnessed during evolution for beneficial purposes, too. In the immune system of vertebrates, genetic diversification and the natural selectability of antibody genes in lymphocytes accommodates the need to recognise and defend against the vast array of potential infectious challenges1.
On the other hand are striking but problematic examples of Darwinian natural selection, including many forms of resistance, that provide the major route of escape for cancer cells from chemotherapy.
For some time after the publication of Peter Nowell’s 1976 review2, most scientists, I suspect, regarded cancer clone evolution as an analogy or parallel with ‘real’, ecosystem-based evolution by Darwinian natural selection; Nowell’s seminal review in fact had made no mention of the latter.
We now appreciate that cancers develop or evolve by somatic cell evolution in the very same sense that an asexual, unicellular species evolves by genetic diversification and selection under ecosystem selective pressures, exogenous, endogenous, etc. 3,4. In the case of cancer, the ecosystem context, or habitat, is the complex architecture and dynamic networks of interactions of cells within tissues and there, multiple selective pressures can be at play influencing cancer clone evolution.
| Exogenous | Genotoxic or toxic damage |
|---|---|
| Endogenous | Intra- and inter-cellular negative feedback signals |
| Architectural constraints (in epithelia) | |
| Metabolic limitations (e.g. hypoxia, acidosis) | |
| Hormonal proliferative drive | |
| Immune restraint or inflammation | |
| Therapeutic | Chemotherapy, immunotherapy |
Our tissues can therefore provide an ‘adaptive’ landscape for cancer cells to travel through in time. The dynamics of this journey of cancer clone evolution are complex and highly variable; the time frame from initiation of a ‘founder’ cancer cell to overt, disseminated malignancy, with a clone size of 109-1012cells, can be anything from a few months to many decades. The tempo is not necessarily slow and steady, as Darwin imagined for evolution in general, but often more akin to what evolutionary biologists call ‘punctuated equilibrium’ with periodic bursts of cancer clonal expansion culminating in metastases as a tipping point.
Most mutant clones that start out with cancerous proclivities never get far 5. But for those that do, the winning ticket is robustness of phenotype (‘survival of the fittest’) including a parasite-like propensity to migrate, colonise and exploit new habitats or tissues distinct from the site of origin. It is these ‘metastases’ that are likely to be lethal to the host.
Another useful evolutionary perspective on cancer is to consider it as an example of atavism – evolutionary reversion6. The emergence of multi-cellularity some 600 million years ago required that the proliferative capacity and independence of single cells was rendered subservient to a collective of cells7. In such social collectives, of which we are a derivative, there is a division of labour and natural selection operates primarily on the whole, multi-cellular organism. The problem, with this otherwise very successful innovation, is the potential for cells to cheat 8. Some cells, and perhaps especially long-lived stem cells, retain an inherent capacity for both extensive proliferation and mutation that can empower escape from the controls. Cancer then is, in effect, a return to a selfish unicellular lifestyle, ignoring or blind to the normal rules of social restraint – and very likely to be to the detriment of the fitness of the whole organism or individual.
In this context, it’s interesting that many of the so-called ‘suppressor’ genes, that restrain cancer, and are often deleted as cancers develop, first appeared ‘co-incidently’ with multi-cellularity itself 9.
One can imagine that such genes, encoding molecules engaged in, say, cell contact relationships, cell cycle restraint or tissue architecture, would have been essential to ensure the integrity and success of multi-cellular organisation.
You might now question why, if cancer is such an inherent risk and a lethal problem for us, hasn’t evolution naturally selected more effectively against it or is there something special about us? Well, evolutionary adaptations have very much legislated against cancer but nothing is perfect.
A major limitation is that the selectable currency in natural selection is fitness for survival and reproduction. Post-reproductively, natural selection will have minimal impact. So there is a particular problem for the rare species such as our own where most individuals survive well beyond their reproductively active years. Cancer is primarily, although not exclusively, a disease linked to post-reproductive ageing.
Another problem is that the pace of evolutionary selection will be slow in any species that reproduces slowly and infrequently. And if that species is sapient, with a rapidly developing social environment and with frequent changes in exposures or ‘lifestyle’ that greatly increase cancer risk, then most likely there will be insufficient time for genetic adaption by natural selection. Nothing in one million years of human evolutionary history could have prepared the human lung for a continual barrage of hot, carcinogenic tar that commenced in the early 20th century with the commercialised manufacture of cigarettes.
In other words, cancer risk in Homo sapiens is a compound of the intrinsic liability of evolution, greatly racheted-up by the mismatch between our complex social lifestyles (and longevity) and our Stone Age genetics6. We’ve moved too fast and live too long for evolutionary processes to adapt us.
Mel
References
1. Boehm T. Design principles of adaptive immune systems. Nat Rev Immunol 2011 May; 11(5): 307-317.
2. Nowell PC. The clonal evolution of tumor cell populations. Science 1976; 194: 23-28.
3. Greaves M, Maley CC. Clonal evolution in cancer. Nature 2012; 481: 306-313.
5. Greaves M. Does everyone develop covert cancer? Nat Rev Cancer 2014; 14: 209-210.
6. Greaves M. Cancer. The Evolutionary Legacy. Oxford University Press: Oxford, 2000.
7. Buss LW. The evolution of individuality. Princeton University Press: Princeton, New Jersey, 1987.