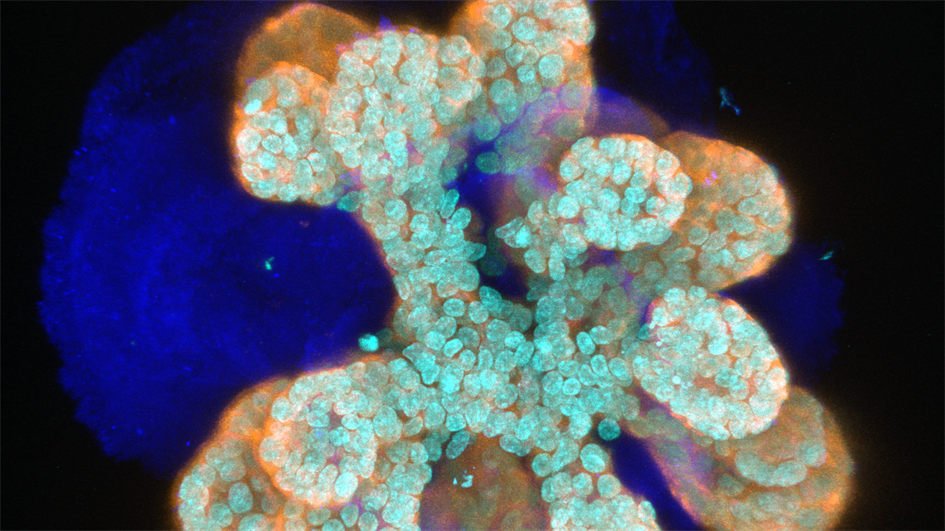
Image: An immunofluorescence image of a mammary organoid in which light blue marks nuclei (DAPI), dark blue marks Collagen IV, and orange marks epithelial cells (cytokeratin 8). Credit: Jorge Almagro Santiago
“Fine, but where is the nipple?” I was speechless. I had spent more than a year figuring out the conditions to finally get my mammary organoids to grow. Ecstatic, I dragged my friend to the microscope only to see the disappointment in her face. “These look nothing like breasts!”
Of course, it’s difficult to live up to the expectations that the word ‘organoid’ may trigger in people’s minds. Some have seen the spectacular images of mini-brains developed a few years ago by biologist Madeline Lancaster and her colleagues in Vienna. In contrast, little groups of mammary gland cells in a petri dish might not sound as exciting. But from a geek’s point of view, I think it is captivating to see how a bunch of isolated cells move and organise into a structure, however simple it might be.
Cells are the building blocks of all the tissues that constitute our organs. Humans are largely just networks of tubes and pipes of different shapes, thicknesses and complexities. Most of these tubes, which form our gut, pancreas, liver, lungs, kidneys, urethra and yes, our mammary glands, are formed of layers of coordinated cells we call epithelia.
The organoid revolution
Over the past century, scientists have proved that different epithelial cells can be removed from their environment and survive in culture conditions. That is to say, stuck to the bottom of plastic dishes and immersed in nutritious soups. While these cultures have brought invaluable advances in our understanding of cell biology, they entail an oversimplification of cells’ behaviour and functions in the organs of our body.
Labs around the world have recently started experimenting with the idea that adding a third dimension to cell cultures can mimic more closely the spatial localisation of cells in our tissues. This can be achieved by growing cells in a blob of a special gelatine. Who could have imagined that cells are so well ‘programmed’ that, just by culturing them in this fancy jelly they would immediately organise and rebuild a small version of the three-dimensional epithelium they came from?
At first sight my mini-breasts might look nothing like actual breasts. However, a detailed examination reveals how similar they are to the tubing that comprises our mammary epithelia. The cells arrange exactly in the same layers, and can undergo the changes women’s mammary glands experience during puberty and pregnancy. Organoids can grow rapidly when we stimulate them with pubertal hormones. The cells in the inner layer of the tube, which we call luminal, divide and produce milk proteins when we create conditions resembling pregnancy. Luminal cells are surrounded by myoepithelial cells, reminiscent of muscles, which contract and squeeze the milk through the ducts and out of the mammary gland. It is lactation in a dish!
A new window into disease
Along with their power to recapitulate physiology, organoids are a great tool to look into pathology. Just as in the mammary epithelia of a person, cancer may arise in an organoid. The meticulously arranged cells fall into sudden disarray. A tissue needs order, and cancer is chaos. It is the difference between having bricks meaningfully assembled or tossed in a random pile. Controlled cell division securing the continuity of the tissue becomes disorganised growth leading to meaningless balls of mess.
Organoids allow us to observe the transition from health to malignancy, which we call transformation. A close inspection under the microscope provides information to answer key questions: What drives transformation? Are all cells potential culprits or are some more ‘malicious’ than others? How do cancer cells talk to each other and to their healthy neighbours? Once we begin to grasp the answers to these questions, all we want to do is keep examining every detail, since even the tiniest event might have phenomenal (or catastrophic) consequences for the tissue.
Indeed, not all cells are at equal risk of becoming cancerous. Stem cells are constantly dividing to substitute older cells, repair damage and maintain our tissues. We owe them our health and the regenerative capacity of our organs. In fact, breast cancer organoids arise from mammary stem cells, but not from other epithelial cells of the breast. But the tireless work of stem cells makes them vulnerable to transformation, and they often initiate cancers.
Growing mini-guts, pancreases, kidneys and more
My job as a PhD student is to closely examine mammary stem cells and look out for potential misconduct and malignant drift. Organoids provide me with the perfect platform for this surveillance. I can identify the stem cells, track their every move and observe any suspicious behaviour indicating transformation, such as a disruption of the tight organisation of the cells or unconstrained proliferation.
Even after a tumour arises, its cells remain heterogeneous. Cancer stem cells are responsible for much of the damaging consequences of the disease. They work incessantly to expand the tumour and invade new tissues. If that was not enough, they often develop strategies to dodge therapy, and can survive hidden in our bodies for years after we believe we have beaten cancer.
Unlike traditional cell culture systems, cancer organoids preserve the heterogeneity of tumours. In this set-up, cancer stem cells have nowhere to hide. I can see in real time where cancer stem cells live, how they defend themselves from chemotherapy and what their vulnerabilities are. By hijacking their anti-chemo defence, I am looking for strategies to re-sensitise and kill them. Eliminating cancer stem cells would mean tackling cancer’s root. The ideal therapy will kill cancer cells and stem cells, and leave the surrounding healthy tissue intact.
For those who might remain unimpressed with the idea of having breasts growing in a dish, it is worth mentioning that today organoids come in all shapes and sizes. Along with mammary and brain organoids, researchers around the globe have developed mini-guts, pancreases, blood vessels, kidneys, prostates, retinas… there is something for everyone. And if no one has developed organoids of your favourite organ, why not put on a lab coat and start working on it?
This piece won the 2021 Mel Greaves Science Writing Prize.
Read more entries from the finalists
 Jorge Almagro Santiago is a molecular biologist from Madrid, Spain, fascinated with advanced imaging as a tool to visualise the cells of our organism in physiology and cancer. He is currently in the last year of his PhD on cancer biology in Professor Axel Behrens' Cancer Stem Cell team.
Jorge Almagro Santiago is a molecular biologist from Madrid, Spain, fascinated with advanced imaging as a tool to visualise the cells of our organism in physiology and cancer. He is currently in the last year of his PhD on cancer biology in Professor Axel Behrens' Cancer Stem Cell team.
He recently moved from the Francis Crick Institute to to the ICR, where he holds an honorary appointment. Before arriving in the UK just over four years ago, he carried out basic research on regulatory mechanisms of inflammation in Vienna, and liver disease and regeneration in Madrid.