Dr Agnieszka Konopacka recently joined The Institute of Cancer Research, London, as Group Leader of the Induced Proximity Therapeutics Group, which sits within the Centre for Protein Degradation in the Division of Cancer Therapeutics. By exploring innovative drug discovery approaches based on targeted protein degradation, her team is looking to find new ways to defeat cancer. Robbie Lockyer spoke with her to learn more.
While DNA stores the cell’s biological information, proteins execute its instructions. They are involved in nearly all cellular functions essential for maintaining the cell’s structure, metabolism, signalling, division, survival and death. When proteins become aberrant – becoming overactive, mutated and problematic – they can drive diseases such as cancer.
Dr Agnieszka Konopacka’s work focuses on the approach of targeted protein degradation, an exciting technology exploiting the cell's natural processes to remove oncogenic proteins, offering a novel way to treat cancer.
Having joined the Division of Cancer Therapeutics in July 2024, Dr Konopacka is bringing fresh expertise to an area where The Institute of Cancer Research (ICR) already has a strong legacy.
From neuroscience to oncology
Dr Konopacka’s career path into cancer research wasn’t linear. Born and raised in Eastern Poland, she gained her PhD at the Mossakowski Medical Research Institute in Warsaw, focusing on neuroscience. A postdoctorate at the University of Bristol followed, during which she honed her expertise in molecular and cell biology, using cutting-edge genetic tools to manipulate and study neurons and glia – cells in the brain and nervous system.
Driven by the potential to turn scientific discoveries into real-world treatments, Dr Konopacka moved to industry in 2014, working on drug discovery projects relating to pain and neurological disorders at Pfizer and oncology projects at Horizon Discovery.
Dr Konopacka explained: "Neurons are one of the most specialised and complex type of cells, with intricate intracellular signalling pathways. Yet, at their core, the molecular processes driving cell homeostasis and survival are very similar between neuroscience and oncology.”
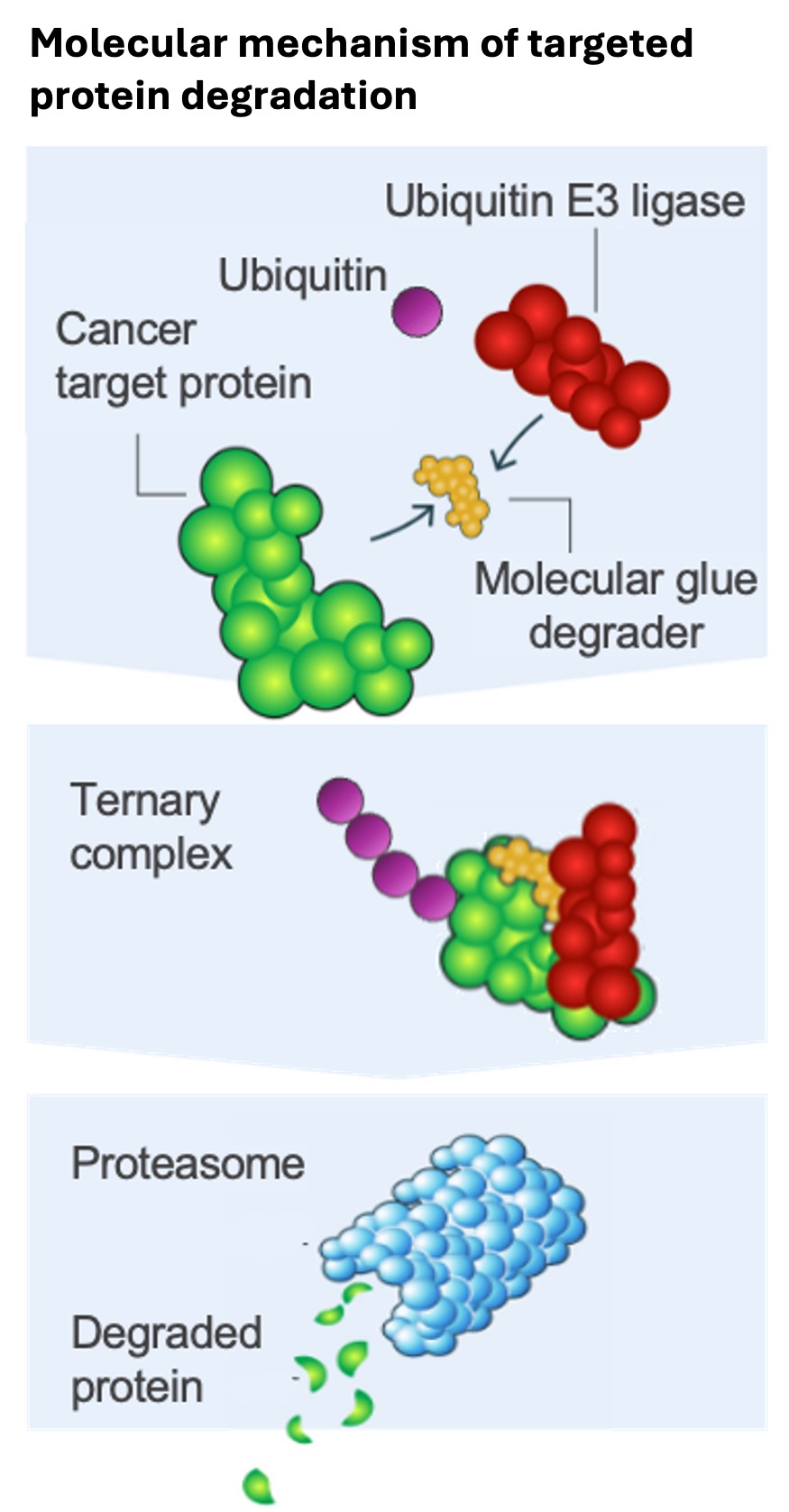
In 2017, Dr Konopacka joined the Protein Degradation Discovery Performance Unit at GlaxoSmithKline (GSK), pioneering the application of novel targeted protein degradation technologies – innovative approaches that target and eliminate oncogenic proteins that tumours rely on for growth and survival – to advance drug discovery. The leading innovations in this field are proteolysis targeting chimeras (PROTACs) and molecular glues, both of which are designed to link target proteins to the proteasome, a molecular machine that facilitates their degradation, acting as a ‘cellular bin’.
Dr Konopacka said: “We were a very talented and dedicated team comprising medicinal chemists, biologists, and in vitro and in vivo pharmacologists. At the time, PROTACs were still in the proof-of-concept phase, and we were all very excited with every bit of positive degradation data.”
In 2024, Dr Konopacka joined the ICR to head up drug discovery biology at the newly formed Centre for Protein Degradation led by Professor Zoran Rankovic. Having long been at the forefront of cancer drug discovery, the ICR already had a long tradition and expertise in targeted protein degradation.
A focus on targeted protein degradation
Oncology is the most obvious area where targeted protein degradation can make a difference because of the opportunity to degrade aberrant and oncogenic proteins. Most existing cancer treatments are based on inhibiting protein function rather than removing the harmful protein altogether. By enabling the selective degradation of specific targets, small molecule drugs offer the potential for safer and more effective cancer therapies.
Dr Konopacka explained: “We are already aware of several aberrant protein targets that are either overexpressed or mutated in cancer. If we can block these proteins of interest or get rid of them through degradation, we can halt cancer growth. Currently available treatment options for cancer are limited due to toxicity, cancer resistance and often insufficient efficacy. PROTACs and molecular glue degraders that can remove the driving oncogene altogether for an extended period can offer better outcomes.
“Protein degradation is based on intracellular processes, and it builds on the cell's natural system for recycling unneeded or defective proteins. This disposal process, which is called the ubiquitin proteasome system, involves tagging aberrant proteins with poly-ubiquitin – a peptide marker or ‘magic protein’ that flags them for destruction by proteasomes.
"Hijacking the natural ubiquitination process allows us to remove aberrant proteins entirely, rather than just inhibiting their activity. By doing so, we hope to achieve better treatment results and overcome key challenges associated with drug resistance and off-target effects.”
Earlier, we touched on PROTACs and molecular glues – tools used in protein degradation. But how exactly do they help attach these ‘magic’ ubiquitin markers to the target proteins needing to be degraded?
PROTACs bind to both a target protein and an enzyme called E3 ubiquitin ligase, which is responsible for recruiting ubiquitin and transferring it to the protein – known as ubiquitination. Once the protein and enzyme are in close contact, the protein becomes ubiquitinated and degraded by the proteasome.
Molecular glues use a similar process, but unlike PROTACs, they are not bifunctional. Instead, they bind E3 ligase or the target protein and induce structural shifts that help the two bind. Once bound, the proteins become ubiquitinated and degraded.
Dr Konopacka explained: “These tools, which we call induced proximity therapeutics, are, excitingly, pushing the boundaries of drug discovery and offering new hope for patients. They allow us to target proteins that were previously considered ‘undruggable.’”
Building a team with a mission
Dr Konopacka is currently assembling the induced proximity biology team at the ICR, bringing together scientists who have experience in protein degradation and drug discovery. The aims are to accelerate progress in this field and build on the ICR’s deep expertise in cancer biology and drug discovery.
Dr Konopacka said: “I’m fortunate to have joined an institute with a strong research history on targeted protein degradation and cancer drug discovery. Together with other groups at the Centre for Protein Degradation, our team is tackling several ambitious projects, including identifying new targets for molecular glues and advancing PROTACs for specific cancer-linked proteins.
“We provide comprehensive biological platforms for target and novel E3 ligase discovery, as well as assay development, screening and the profiling of molecular glue degraders and PROTACs. We are aiming to improve drug selectivity, reduce toxicity and potentially spare healthy tissue by focusing on proteins or pathways unique to cancer cells.”
A vision for the future
Dr Konopacka believes induced proximity therapeutics have the potential to transform cancer therapy. By employing the cell's natural protein degradation systems, the technology may offer a competitive edge due to targeting proteins that have been considered difficult to drug by traditional approaches such as inhibitors.
Dr Konopacka said: “The hope is to deliver treatment that is more efficient and minimises side effects. As these PROTACs and molecular glues molecules can degrade multiple copies of target proteins, we can use smaller doses, which reduces the risk of both toxicity and treatment resistance.
“I’m enthusiastic about this technology because it’s incredibly clever, and we can see how we’re making a difference – not only for patients but also for the scientific community, as our degraders can be used as important tools for target validation and basic biology. These achievements are very rewarding for us at the ICR.
“With our interdisciplinary expertise and extensive collaborations with other groups at the ICR and drug discovery industry, I believe we’re exceptionally positioned to drive innovation in targeted protein degradation drug discovery and cancer research. As I settle into my new role, I’m excited about the path ahead.”