Drug Target Discovery Group
Professor Tutt's group aims to identify and validate new molecular targets and to develop drugs through the process of hit generation, lead identification and candidate selection.
Industrial partnership opportunities with this group
Opportunity: Cancer biomarker for predicting response to drugs targeting mitotic checkpoint kinases and cell division
Commissioner: Professor Andrew Tutt, Professor Chris Lord, Professor Jonathon Pines
Recent discoveries from this group
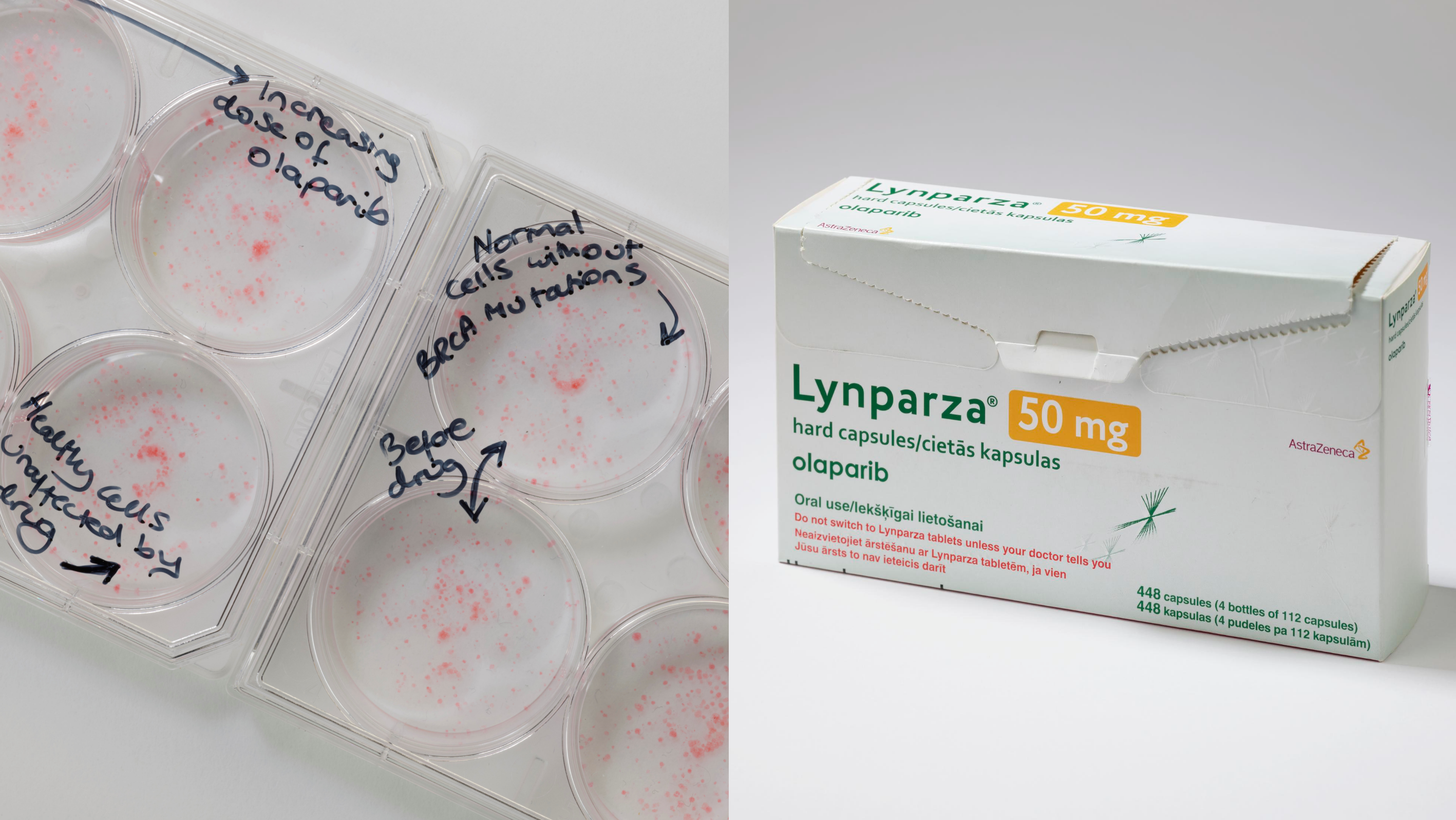
ICR welcomes NICE recommendation of olaparib for advanced breast cancer
The Institute of Cancer Research, London, strongly welcomes the decision by NICE to recommend that the targeted drug olaparib can be used for locally advanced or metastatic breast cancer.
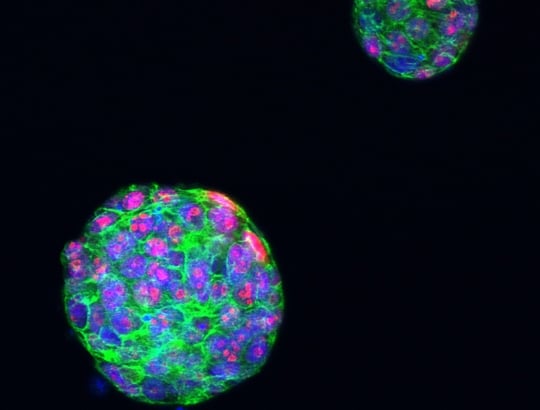
Researchers identify what drives PARP inhibitor resistance in advanced breast cancer
Researchers at The Institute of Cancer Research, London, have increased our understanding of how a cancer drug called a PARP inhibitor stops working in women with breast cancer that has spread. This research could ultimately help predict who’s more likely to respond to these drugs, and could lead to more effective ways to treat the disease.
-(3)-4088-x-3076.jpg?sfvrsn=81e087bc_2)
ICR urges continued negotiation after NICE rejection of olaparib for early breast cancer
The Institute of Cancer Research, London, is urging NHS-England, NICE and pharmaceutical company AstraZeneca to continue discussions after the disappointing decision not to recommend targeted drug olaparib for women with early-stage, high-risk, inherited breast cancer.
-547x410.jpg?sfvrsn=5e98f2c_2)
Olaparib for high-risk, early-stage breast cancer approved in Europe
The Institute of Cancer Research, London, strongly welcomes the news that the precision medicine olaparib has been approved in Europe for people with high-risk, early-stage breast cancer who have inherited faults in their BRCA1 or BRCA2 genes.