
PIVOTALboost
PIVOTALboost investigates different ways of giving radiotherapy to people with prostate cancer.
Disease site: Prostate cancer
Status: In follow-up
What is the study about?
PIVOTALboost investigates different ways of giving radiotherapy to people with prostate cancer.
Prostate cancer radiotherapy is normally targeted at the prostate gland. Giving radiotherapy to lymph glands in the pelvis, or giving a higher dose (boost) of radiotherapy to the prostate, may help reduce the chance of cancer returning after treatment.
PIVOTALboost uses modern radiotherapy techniques to boost radiotherapy treatment for people with prostate cancer. Results will be used to improve treatment for people with prostate cancer in future.
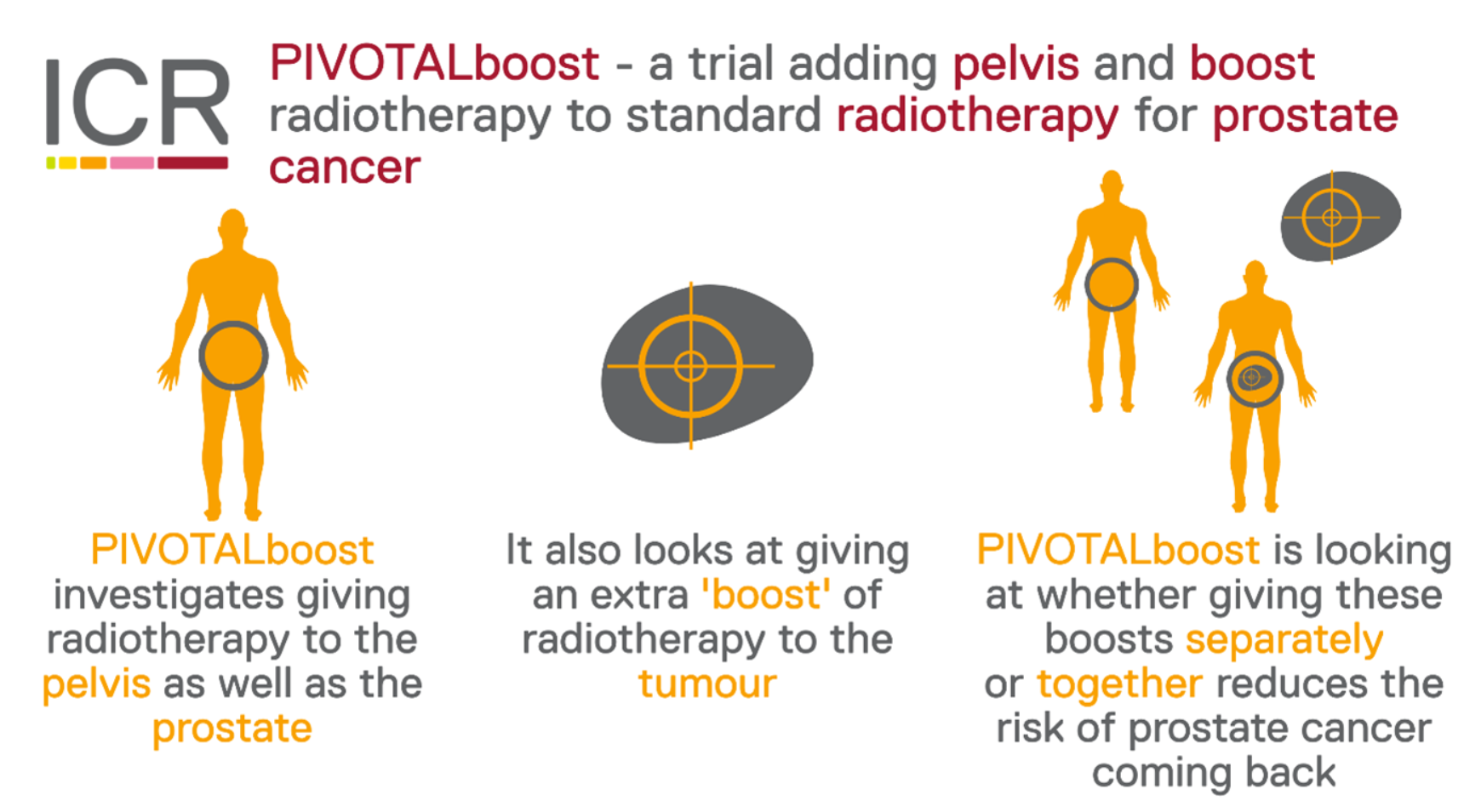
Image: PIVOTALboost - a trial adding pelvis and boost radiotherapy to standard radiotherapy for prostate cancer. PIVOTALboost investigates giving radiotherapy to the pelvis as well as the prostate. It also looks at giving an extra boost of radiotherapy to the tumour. PIVOTALboost is looking at whether giving these boost separately or together reduces the risk of prostate cancer coming back.
Who is included in the study?
PIVOTALboost includes 2,229 people with prostate cancer that has a medium or high risk of returning after treatment and has not spread elsewhere in the body. Everyone joining the study will start hormone therapy before receiving radiotherapy. Participants are enrolled at NHS hospitals across the UK.
What are the study treatments?
Everyone who joins the trial will be in one of four treatment groups:
- Prostate radiotherapy (standard treatment)
- Prostate and pelvis radiotherapy (enrolment to this group closed in 2022)
- Prostate radiotherapy with boost to the cancer within the prostate or the whole prostate gland
- Prostate and pelvis radiotherapy with boost to the cancer within the prostate or the whole prostate gland
Radiotherapy will be given every week day for four weeks.
People in the boost groups will have their boost given either:
- With a temporary implant put into the prostate before or after radiotherapy
- With radiotherapy at the same time as the rest of their treatment
Participants have regular check ups during and after their treatment and we collect information about how they are getting on until the study is completed.
Further information for participants
Patient Information Sheet (IMRT Boost)
Patient Information Sheet (HDR Boost)
Patient Information Sheet (HDR Whole Gland)
A detailed summary is available on Cancer Research UK's website
Recruitment completion thank you newsletter
Further information for healthcare professionals
Contact details and regulatory information
Chief Investigator: Professor Isabel Syndikus, Clatterbridge Cancer Centre
ICR-CTSU scientific lead: Professor Emma Hall
Trial management contact: [email protected]
Sponsor: The Institute of Cancer Research
Funding: Cancer Research UK
Publications and presentations
Review of data quality following a transition to telephone follow up in phase III multicentre clinical trials as a result of the COVID-19 pandemic. V. Hinder, L. Philipps, M. Mahmud, A. Omar, S.Brown, C.Cruickshank, S. Burnett, I. Syndikus, N. Van As, A. Tree, E. Hall (NCRI Oct 2021)
PIVOTALboost: A phase III randomised controlled trial of prostate and pelvis versus prostate alone radiotherapy with or without prostate boost (CRUK/16/018). Syndikus I, Cruickshank C, Staffurth J, Tree A, Henry A, Naismith O, Mayles H, Snelson N, Hassan S, Brown S, Porta N, Griffin C, Hall E. Clinical & Translational Radiation Oncology (2020)
Successful Implementation of Treatment Planning Trial QA for PIVOTALboost Trial. Mayles H, Naismith O, Snelson N, Syndikus I, on behalf of PIVOTALboost TMG. Radiotherapy and Oncology (2020) 152
PIVOTALboost Trial Contouring RTQA. Syndikus I, Tree A, Staffurth J, Mayles H, Naismith O, Snelson N, Montazeri A, Hall E. Abstract submitted to ASCO 2020 Genitourinary Cancers Symposium. Journal of Clinical Oncology 38, no. 6_suppl (February 20, 2020) 373-373.February 13-15, 2020).
Updating recruitment forecasts via simulations to explore impact of availability of experimental arms in the phase III PIVOTALboost trial. Porta N, Hinder V, Griffin C, Tree A, Staffurth J, Henry A, Hassan S, Brown S, Cruickshank C, Syndikus I, Hall E. Trials 2019, 20(Suppl 1):P-22
Successful collaboration between a Clinical Trial Unit (CTU) and the National Radiotherapy Trials Quality Assurance (RTTQA) Group to expedite opening a complex phase III trial (PIVOTALboost). Cruickshank C, Mayles H, Naismith O, Snelson N, Tree A, Staffurth J, Henry A, Hassan S, Brown S, Syndikus I, Hall E. Trials 2019, 20(Suppl 1):P-21
Pre-trial outlining quality assurance in PIVOTALboost. Evans E, Mayles H, Naismith O, Hall E, Tree A, Staffurth J, Syndikus I. ASCO 2019 Genitourinary Cancers Symposium. Journal of Clinical Oncology 37, no. 7_suppl (March 01, 2019) 50-50.
Use of simulations to explore recruitment and the impact of late availability of experimental arms in the PIVOTALboost trial. Porta N, Griffin C, Syndikus I, Staffurth J, Hoskin P, Dearnaley D, Henry A, Carey B, Tree A, Lewis R, Hall E. Trials 2017;18 (S1), P51. 10).

