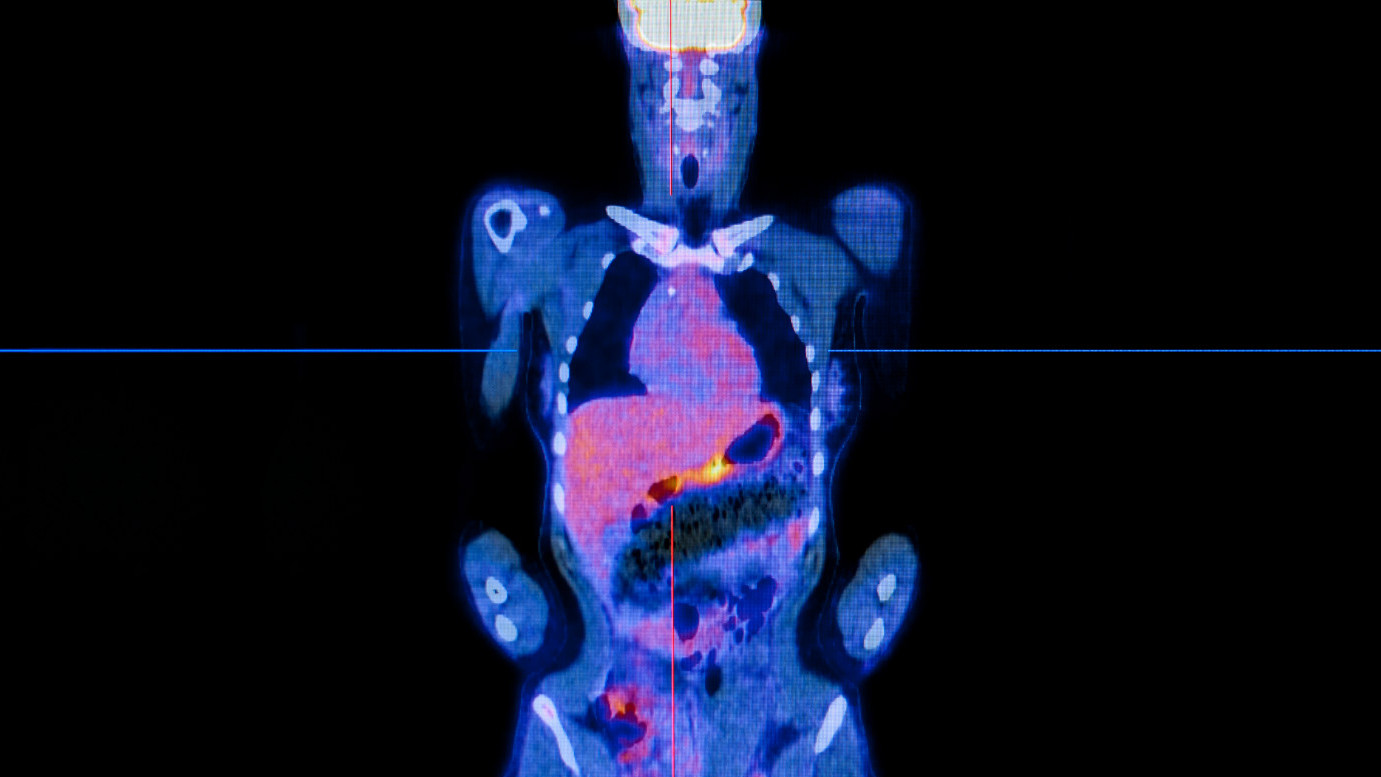
Scans of patients have revolutionised modern medicine – allowing doctors to peer inside the body without making a single incision. They allow doctors to trace the growth and spread of cancers, and to accurately target treatments such as surgery and radiotherapy.
But standard imaging techniques have their limitations. They can only reveal the location and size of the tumours, and it can be difficult to tell tumours apart from the surrounding healthy tissue. So researchers at The Institute of Cancer Research, London, have been active in development new imaging techniques, including ones that use positron emission tomography (PET) – a modern technique that employs radioactive probes to ‘spy’ on a cancer’s behaviour, including metabolism. PET detects the biochemical processes that distinguish cancer cells from normal cells and can reveal even miniscule tumours within healthy tissues.
PET imaging had its beginnings in the early part of the 20th century when the German chemist and physician Otto Warburg discovered that when sea urchin eggs were fertilised, they underwent rapid growth and their metabolic rates increased dramatically. He noticed similarities between the fertilised sea urchin eggs and cancer cells, and found that cancer cells too break down sugar at a much faster rate than normal cells. We now know that cancer cells often undergo extensive metabolic reprogramming, elevating their metabolic rate and nutrient uptake in order to meet the demands of uncontrolled growth. Many cancer cells become addicted to nutrients such as glucose and glutamine for their survival.
But it wasn’t until the 1970s that chemists Tatsuo Ido and Alfred Wolf created the first PET tracer. They inserted radioactive fluorine into a glucose molecule to produce a labelled fluorodeoxyglucose (FDG) tracer. FDG is closely related to glucose and is easily taken up from the blood stream by cells, but can’t be fully broken down by them, so it remains trapped inside the cells emitting radioactive signals that can be detected. In the past few decades, FDG-labelled PET has come to the forefront in the assessment of the molecular pathology of cancer in humans, and is currently a well-established procedure in the diagnosis of patients with a wide range of cancer types. But there is a continual search for newer and better radiotracers to spy on cancers’ metabolic processes.
Dr Graham Smith, who heads the PET Radiochemistry Team at the ICR, is developing new imaging agents that can reveal further insights into tumour metabolism, and can help assess how a patient is responding to treatment.
One of the new tracers under the team’s development uses glutamine, an abundant and versatile sugar that plays an important part in cancer cells’ biological processes. By using radiolabelled glutamine as a tracer, researchers are able to investigate a different area of cancer metabolism from that picked up with FDG. “Some cancers, for instance prostate cancer, lack the high rates of glucose uptake necessary for using an FDG tracer,” explains Dr Smith. “Also, the reliability of FDG is limited when the tumour is adjacent to normal tissues where FDG is concentrated, for instance the brain or the bladder. Glutamine consumption and excretion is lower in these tissues, so glutamine-based PET could provide improved contrast between the tumour and normal tissue.”
Dr Smith is also developing glutamine tracers to probe resistance and response to treatment. He is working with Dr Yuen-Li Chung in the Magnetic Resonance Team, who will be looking at glutamine metabolism using PET and magnetic resonance spectrometry – a type of imaging scan. “Dr Chung wants to study cancer metabolism and its response following treatment with targeted therapies. Using our new tracers, she will be able to assess tumour response and identify the emergence of resistant disease.”
There are still large gaps in our understanding of which metabolic pathways are altered in cancer, and how these changes affect the way a cancer behaves. But the ability to spy on cancer using radiotracers has started to fill in these gaps, and is revealing more and more of cancer’s secrets.