Throughout history, humans have endeavoured to see things that were too small to be perceived with the naked eye in order to understand our complex world.
The first magnifier constructed for scientific purposes was believed to have been designed by the English philosopher Roger Bacon sometime during the 13th Century. But it wasn’t until the 17th Century that Antonie van Leeuwenhoek — a Dutch draper with boundless curiosity — became the first person to make and use a real microscope. He wanted to see the quality of thread in greater detail than his then-current magnifying lenses allowed. His discovery opened a gateway to a world teaming with microscopic life.
Traditional microscopy uses light and allows researchers to view only a few structures inside cells. If scientists want to view the internal workings of a cell during complex processes like cell division, things get trickier. Living tissues are very sensitive to light, and do not behave normally if the light levels are too high.
This, combined with the need to image specific processes within the cells more quickly and in ever greater detail, has led to the development of advanced techniques.
'Optical tweezers'
Recently a new type of microscope has been developed called the lattice light sheet microscope, and The Institute of Cancer Research, London, will be the first research organisation in the UK to receive this high-tech instrument.
Invented by the Nobel laureate Dr Eric Betzig, the microscope uses ‘Bessel beams’, which don’t behave in the same way as light. Bessel beams are already used in science as optical tweezers — highly focused laser beams to provide an attractive or repulsive force. They are even being studied for use as tractor beams, an invisible force used to move large objects seemingly without effort.
Now, scientists are using this technology to watch the tiny proteins that move within a cell, to see how they are activated and elucidate what drug molecules might stop their activity to help defeat cancer. The beams are so narrow that they reveal details unseen using normal light, and can be swept across the cell sample whilst rapidly switching them on and off to avoid damaging the cell.
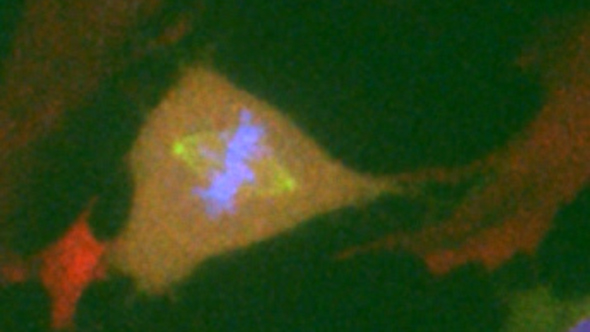
Normal diploid human Retinal Pigment Epithelial (RPE1) cell in which fluorescent proteins have been used to tag the endogenous Cyclin B1 (Green) and Mad2 (Red) proteins, and a tagged exogenous CENP-A (Blue) introduced (image captured from clip in video above. Credit: Philippe Collin, Oxana Nashchekina and Mark Jackman)
Professor Jonathon Pines, Head of the Division of Cancer Biology at the ICR, plans to use lattice light sheet microscopy to reveal how cells divide, in particular how the machinery that controls cell division is regulated in space and time.
His team are focusing on how both normal and cancer cells trigger and control mitosis — the process of cell division.
Investigating new drug targets
To investigate the mechanisms controlling a process as highly dynamic as mitosis, the lab has developed a number of powerful techniques using live cells.
Using probes that detect specific enzymes called cyclin-dependent kinases, which are essential to cell division, Professor Pines’s team is investigating how and when these enzymes are turned on, forcing the cell into mitosis.
Professor Pines says: “This event is important from a cancer perspective, because if a cell enters mitosis at the wrong time this can lead to chromosome damage, and to mutations in the DNA being inherited by one of the daughter cells. Because cyclin-dependent kinases have important roles in regulating cell growth and division, they are being investigated as targets for anti-cancer drugs.”
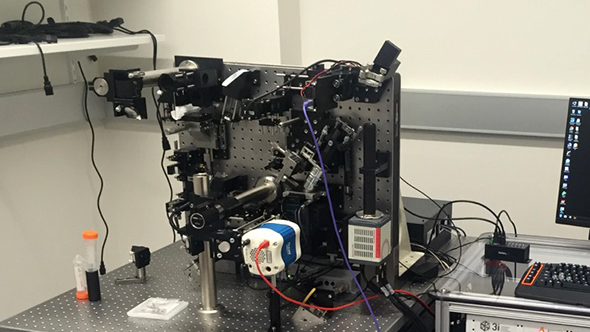
The lattice light sheet microscope (photo: Intelligent Imaging)
Researchers are also investigating how dividing cells are able to ensure that the two daughter cells receive an equal and identical set of chromosomes. If this goes wrong, and one cell receives an extra or a damaged chromosome, this can lead to cancer.
In fact, this is a hallmark of some of the most aggressive forms of cancer. Professor Pines explains: “We know that cells safeguard against these types of problems by having specific checkpoints that block the next stage in the cell division process when something goes wrong. When the cell confirms that everything is in order, it is only then that the proteins are destroyed. What we want to know is: how does the cell destroy the right protein at the right time?”
Powerful new tools
Building such a detailed picture of mitosis has given drug discovery a new lease of life. “Drugs that target mitotic events, such as the highly successful taxanes, have been available for some time,” explains Professor Pines. “But they have unwanted side-effects and resistance to treatment gradually appears. We aim to design the next generation of drugs that will target mitosis in a better and safer way than currently available treatment.”
Understanding the machinery of mitosis has increased dramatically over the last few years thanks to powerful new microscopes that have enabled biologists to focus on the processes that sustain life.
The gateway to a microscopic world that was opened over 350 years ago by van Leeuwenhoek is now wide open, and is helping us to understand not only what drives life, but also what can cause cancer.
A gift from you will help us carry out pioneering research using the new lattice light sheet microscope, and lead us to discover more effective treatments.