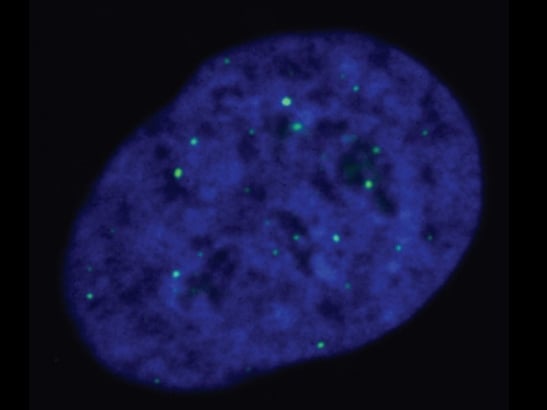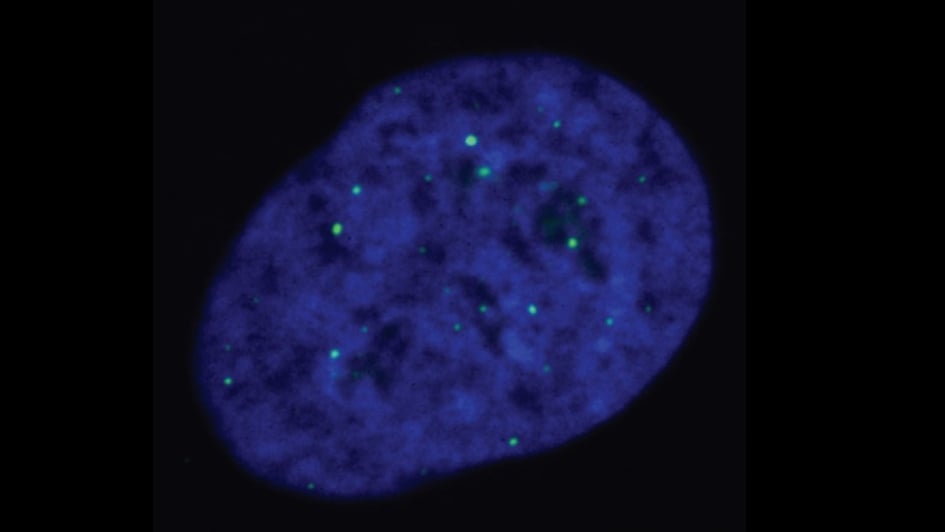
Image: ALT-reliant cells, with telomeres being marked by the green dots. Credits: Cancer and Genome Stability Team at the ICR
A new study has provided insights into an important biological mechanism that supports survival of aggressive, hard-to-treat cancers – and in the process, uncovered fascinating new information about how cells divide and grow.
In the new study, led by scientists at The Institute of Cancer Research and published in Nature Communications, researchers identify a new cellular role of a protein called EXD2 nuclease and, importantly, its role in the so-called ALT pathway.
The study, which was funded by the ICR itself – which is a charity as well as a cancer research institute – and Cancer Research UK, also provides potential new targets, against which cancer drugs could be developed for patients who currently have limited therapeutic options.
Telomeres and the ALT pathway
ALT is short for alternative lengthening of telomeres, which have an incredibly important function in cells. They act like a protective cap during normal cell division but shorten every time a cell divides, which makes cell death inevitable over time.
This means that to survive and proliferate, cancer cells must subvert this natural process of telomere shortening, maintaining the length of their telomeres.
They achieve this through two different mechanisms: either through activation of telomerase – an enzyme that promotes lengthening of telomeres – or via the ALT process.
The ALT pathway is known to support survival in 10 to 15 per cent of cancers. There is very limited knowledge about how ALT works – and yet it’s one of the absolutely fundamental pathways in those cancers.
Major role of EXD2 nuclease
Building on previous work that uncovered a key role for EXD2 nuclease in DNA replication, in this study the research team focused specifically on EXD2 in ALT-reliant cancers. In this new study they established that EXD2 is essential to promote telomere maintenance via a process called break-induced replication, and discovered that the loss of EXD2 in ALT cells resulted in telomere shortening.
Importantly, the study also found that EXD2 depletion killed ALT-dependent cancer cells if combined with the loss of other DNA repair proteins – such as BLM, DNA2 and POLD3.
Therefore, this work provides a proof-of-concept that targeting EXD2 nuclease in addition to either one of those three gene products, could be a new strategy to eradicate tumours relying on the ALT mechanism for survival.
Potential targeted therapy for hard-to-treat cancers
Professor Wojciech Niedzwiedz, Leader of the Genome Instability and Cancer group at The Institute of Cancer Research, London, said:
“Some 10 to 15 per cent of cancers support cell proliferation via the ALT mechanism, including up to 50 per cent of hard-to-treat osteosarcomas, soft tissue sarcomas and primary brain tumours – including childhood brain tumours. These ALT-reliant cancers are highly aggressive and there are very limited treatment options. Understanding how these tumours maintain telomeres to sustain unlimited growth, at the molecular level, is therefore essential to help develop new therapies.
“Our study expands on our understanding of how cancer cells look after their telomeres in order to achieve immortality. Importantly, it also uncovers new synthetic lethal interactions within the ALT pathway and as such, paves the way for the development of new anti-cancer therapies.’’
Dr Ronan Broderick, lead author of the study and part of the Genome Instability and Cancer group at the ICR, added:
‘’A lot of work in this study was made possible by the equipment provided by the Light Microscopy Facility in Chelsea. Dr Tina Daviter, Head of Core Research Facilities at the ICR, and Dr Kai Betteridge, Manager of the Light Microscopy Facility, have developed amazing state-of-the art microscopy at the ICR which is essential in analysing the critical mechanisms of cell division, to identify potential cancer drug targets.’’
Future research
Professor Niedzwiedz is aiming to further develop aspects of his programme focusing on targeting EXD2 nuclease in cancer in collaboration with industry.