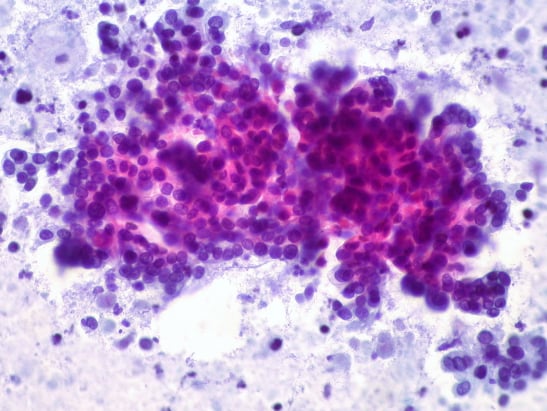
Image: Pancreatic adenocarcinoma (left) vs. normal ductal epithelium (right). Credit: Ed Uthman, CC BY 2.0
By the time a pancreatic tumour begins to cause symptoms, it is usually pretty advanced, since many of the tumours are ‘silent’.
Dr Anguraj Sadanandam, a team leader in the Division of Molecular Pathology at The Institute of Cancer Research, works on two types of pancreatic tumours: neuroendocrine tumours and adenocarcinomas.
Neuroendocrine tumours are exceptionally rare, making up just one to three per cent of all pancreatic cancers.
There are two types of pancreatic neuroendocrine tumour – functional and non-functional. Functional tumours are tumours which produce hormones, which means they’re more likely to produce noticeable symptoms.
The majority of pancreatic neuroendocrine tumours, however, are non-functional, meaning they don’t produce any hormones. These tumours grow silently, and without hormone production, they usually don’t produce symptoms until they have advanced to a large size.
At this point, the tumours are usually inoperable, and treatment options are limited to palliative care.
In fact, pancreatic tumours are often diagnosed only when they have spread to other organs, and begin to cause symptoms there. Some 60% of patients diagnosed with non-functional pancreatic cancer have metastases at diagnosis – half of these in the liver.
“Diversity causes difficulty in clinical practice” – so says Dr Anguraj Sadanandam.
He recently published a review on the molecular biology of pancreatic neuroendocrine tumours.
“There is a clear unmet need when it comes to pancreatic neuroendocrine cancer – we know that patients diagnosed with pancreatic tumours are not seeing the same improvements in treatment that we see for other cancer types.”
Our researchers are dedicated to tackling cancers that continue to have very poor survival rates – such as pancreatic cancer.
Categorising the problem
Pancreatic neuroendocrine tumours are divided into grades by the World Health Organisation based on features of the cells that are common when we look down the microscope.
When cells divide, they make a complete copy of all of their chromosomes.
Under ordinary circumstances, a cell keeps all of its genetic material wound up and compact inside of the nucleus – the control centre of the cell. In order to divide, the cell unwinds all of its chromosomes and unzips them to make copies.
By using special staining, scientists are able to assess how many cells have visible chromosomes. Visible chromosomes give a good indication that the cell is in the middle of dividing.
If lots of cells show signs of being in the middle of dividing, scientists can get an indication of how fast the tumour is growing and how aggressive it is. More cells making copies means more growth and a highly aggressive tumour.
Combining this information about cell division with other visible changes to cells results in pancreatic tumours being divided into three grades.
Grade 1 tumours have the best prognosis – patients have a median overall survival of 12 years with this type of tumour.
Median overall survival refers to the length of time from either the date of diagnosis or the start of treatment for a disease that half of patients in a group are still alive.
Grade 3 tumours have the worst prognosis, with a median overall survival of just 10 months.
The patient perspective
Roy Bowdery was diagnosed with pancreatic cancer in April 2014 following a prolonged period of pain in his stomach and back.
His pancreatic cancer was an adenocarcinoma – these cancers have different biology from neuroendocrine tumours, but survival compared to other more common cancers is also relatively poor.
Although one-year survival rates for pancreatic cancers have been improving in recent years, there hasn’t been much of an improvement in five and 10-year survival rates since the 1970s.
Despite repeated trips to his local GP, it took several weeks before Roy was sent for a CT scan as part of a local bowel cancer screening programme, and it was only then that doctors discovered the tumour on his pancreas.
Fortunately, Roy qualified for the Whipple procedure – a 10-hour surgery to remove the lower half of the stomach, duodenum, gall bladder, bile duct and the head of the pancreas.
This was followed up with six months of chemotherapy.
Roy now lives cancer-free, but goes for check ups every six months. He knows he's one of the lucky few. One in four people die within the first month of diagnosis and only 1 per cent of patients survive 10 years.
"Pancreatic cancer is a really brutal type of cancer," Roy explains. "It's the most fatal of all common cancers. I've just gone over the five year mark, and now my aim is to get into the one per cent."
Since his diagnosis, Roy has been actively campaigning to change the journey for others in the future.
"The figures haven't improved in my five years, and they haven't even improved in the last 40 years. There needs to be more research, and there needs to be a quick, easy diagnostic test to catch the cancer early, so more people get a chance at surgery and get a chance to survive”.
We are now poised to outsmart cancer with the world’s first anti-evolution 'Darwinian' drug discovery programme, in which we will focus on understanding, anticipating and overcoming cancer evolution, and preventing drug resistance.
Improving diagnosis
Although the World Health Organisations’ grading of tumours is useful in that they give some indication of prognosis, there are substantial differences in tumours within the same grade, and this makes personalised treatment for these tumours hugely challenging.
Dr Sadanandam published a major paper five years ago which divided pancreatic neuroendocrine tumours into molecular subtypes.
The study recreated pancreatic cancer in mice, and together with data from human cancers the researchers were able to classify the tumours in three different ways.
Untangling the complexities of the cancer and categorising the tumours can help when it comes to making decisions about treatment - the aim is always to personalise treatment as much as possible for the specific tumour and the specific patient in front of you.
In other types of cancer, recent research has drastically improved our ability to give a precision diagnosis to each patient, which not only improves the accuracy of the information we can give to a patient about how their cancer is likely to progress, but also allows clinicians to pick the best possible treatments.
For example, recent research from the ICR showed that testing men for faults in DNA repair genes in their tumours could identify those most likely to respond to a new type of “search-and-destroy” treatment.
The treatment seeks out a particular molecule called PSMA (prostate-specific membrane antigen) on the surface of prostate cancer cells and uses a radioactive particle to kill the cells.
This type of approach - finding something unique to a particular cancer type, identifying it and targeting treatments against it – is the next big challenge when it comes to pancreatic cancer.
Dr Sadanandam explains:
“There is a clear unmet clinical need for novel prognostic and predictive biomarkers to complement the World Health Organisations’ grading system.
“We need more detailed information with which we can categorise tumours to guide us in determining overall survival rates and to support individualised treatment decisions for patients.”
Learning lessons from other cancers
In order to bring pancreatic cancers to a level playing field with other cancers, research focusing on comparisons is key.
By examining the similarities pancreatic cancers have to other types of cancer, scientists can begin to unravel the specific cell changes that make the tumours so aggressive and difficult to pin down.
In order to bring pancreatic cancers to a level playing field other cancers, research focusing on comparisons is key. That’s an approach Dr Sadanandam is taking in an ongoing study which is comparing the similarities pancreatic and other cancers have to colon cancers.
By classifying and reclassifying cancers into different subtypes, the problem becomes easier to tackle – like categorising your belongings before tackling a big spring clean.
Other ICR researchers are taking the same approach – Professor Chris Lord, Deputy Head of the Division of Breast Cancer Research at the ICR works with PrecisionPanc, a research platform which has collaborators from across the cancer research spectrum.
They are translating basic scientific discoveries into the clinic by looking at the genetics of pancreatic cancers, and developing biomarkers which help doctors understand the roadmap of a patient’s cancer. They are also working hard to develop new treatments to tackle the tumours.
There is a long way to go before the playing field is leveled, but further research will pave the way for smarter, kinder treatments.