Ovarian cancer is the sixth most common cancer in the UK, with around 7,500 new cases annually, and globally more than 300,000 women are diagnosed each year. This cancer is particularly difficult to catch in its early stages because its symptoms – which include bloating, abdominal pain, and changes in appetite – are often mistaken for a less serious condition. As a result, the majority of ovarian cancer cases are only discovered once the disease is advanced, when treatment options are limited and outcomes more uncertain.
It only takes a moment to make a gift, but the impact of your donation can last a lifetime. Please donate today to help more women with cancer live longer, better lives.

Image: Professor Udai Banerji
The importance of research
The Institute of Cancer Research (ICR), which has played a key role in ovarian cancer research for decades, is committed to changing this and is tackling ovarian cancer’s specific challenges.
Professor Udai Banerji, Co-Director of the Drug Development Unit, Professor of Molecular Cancer Pharmacology at the ICR and Consultant Oncologist at The Royal Marsden NHS Foundation Trust, is among those working to find innovative ways to treat ovarian cancer.
“At the start of my career,” says Professor Banerji, “I had made up my mind to work in drug development and had to choose between working in cancer, infectious diseases or dementia. Choosing cancer was an easy decision for me because I saw a huge amount of research potential there.”
Through his work at the Drug Development Unit, Professor Banerji is contributing to the ICR’s efforts to make significant strides in ovarian cancer treatment. His research highlights the importance of developing tailored therapies that meet the specific needs of different ovarian cancer types and demonstrates the impact of targeted treatments in addressing this complex disease.
Types of ovarian cancer
- High-grade serous ovarian cancer is the most common and aggressive form, making up approximately 60-70 per cent of epithelial ovarian cancers. It is characterised by rapidly growing tumours in the fallopian tubes.
- Clear cellular ovarian cancer is a rare subtype often linked to endometriosis, representing 10 per cent of epithelial ovarian cancers. It typically shows resistance to chemotherapy.
- Low-grade ovarian cancer is a less common and slower-growing form of ovarian cancer, representing 5-10 per cent of epithelial ovarian cancers. It usually arises from benign serous tumours.
- There are also several less common subtypes including, but not limited to, mucinous ovarian cancer, endometrioid ovarian cancer, transitional cell carcinoma, undifferentiated carcinoma and germ cell ovarian cancer.
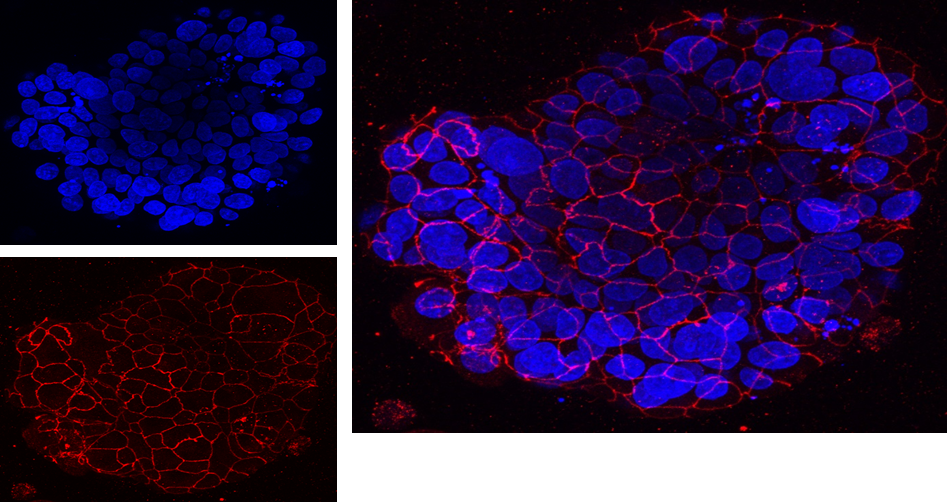
Image: Organoid or ‘live mini tumours’ of high grade serous ovarian cancer grown in ICR laboratories from samples of cancer cells isolated from patients. These live mini tumours are used for a host of research activities including studying the biology of ovarian cancer, discovering new cancer targets or testing new anticancer drugs on patient derived tumour.
Targeted drug development
Professor Banerji is actively involved in developing new drugs tailored to the different types of ovarian cancer.
Idetrexed: A targeted ‘Trojan horse’
Idetrexed is a drug that effectively acts like a ‘Trojan horse’. About a third of high-grade serous ovarian cancers express a protein called the folate receptor. This protein is tricked to bind to idetrexed, part of which is designed to mimic vitamin B9 (folic acid). The folate receptor transports the entire drug into the cancer cell thinking it is folic acid. However, part of the drug is actually a chemotherapy agent known as a thymidylate synthase inhibitor. This ensures that the cancer cells expressing the folate receptor are specifically targeted, while non-cancerous cells remain largely unaffected.
“As cancer cells have high levels of folate receptors compared to non-cancerous cells, idetrexed is selectively taken up by cancer cells, resulting in fewer side effects compared to chemotherapy. This makes it a smarter, kinder treatment,” says Professor Banerji.
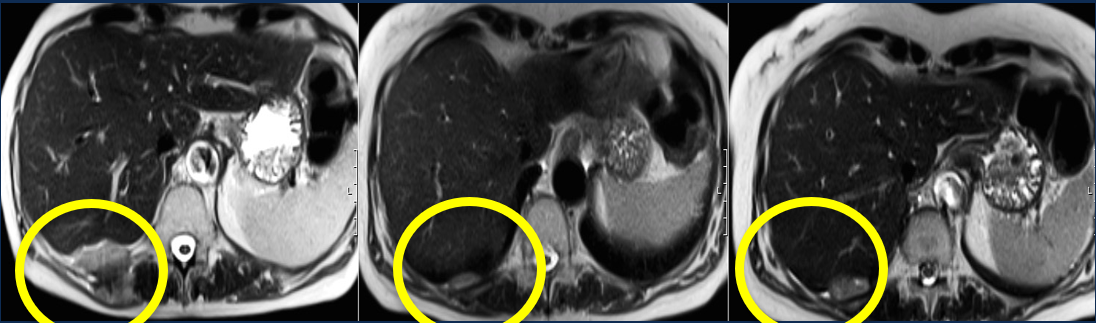
Image: MRI of tumour reducing in size over time (10 months) after given idetrexed. Presented by Professor Udai Banerji at ASCO General Meeting 2017
NXP800: Addressing rare and treatment-resistant cancers
NXP800, a highly innovative drug discovered by the ICR, is designed to treat clear cell ovarian cancer, an often more resistant form of the disease with limited treatment options. The drug, which was discovered through pioneering research led by Professor Paul Workman at the Centre for Cancer Drug Discovery at the ICR, activates an enzyme that regulates cellular stress response. NXP800 targets pathways that cancer cells rely on to cope with the stress of their rapid growth and adverse tumour environments. Once these shields are down, the cells become vulnerable, have delayed growth and are susceptible to further treatment. Professor Workman’s team showed the drug was effective in models of clear cell ovarian cancer, which do not respond well to chemotherapy and often have a mutation in the ARID1A gene.
Professor Banerji played a leading role in the phase I trial of NXP800, which aimed to determine the appropriate dose and schedule for this drug during its first testing in people. Alongside studying and managing the side effects of NXP800, these trials provided critical insights into how the drug functioned in the human body. Professor Banerji’s research has demonstrated the same biological changes caused by NXP800 in laboratory experiments, in both the blood and tumour of patients, validating Professor Workman’s findings. After the results of the phase I trial for NXP800, Professor Banerji and his team were able to recommend a dosage, which is now being evaluated in patients with clear cellular ovarian cancer in an expanded international phase Ib trial being run by Professor Susana Banerjee at The Royal Marsden and the ICR.
“We work closely with The Royal Marsden, and a lot of the clinical trials performed in the Drug Development Unit go on to become phase III trials there. The bench-to-bedside journey is remarkable. We undertake the pioneering drug discovery and innovative early development before drugs are transitioned to world leading tumour type specific units at The Royal Marsden. The ICR is one of the very few places that has this breadth of expertise,” says Professor Banerji.
Combination therapy: avutometinib and defactinib
In 2019, Professor Banerji and his research team started a clinical trial that combined the drugs avutometinib and defactinib, which are designed to block signals that encourage cancer cells to grow. The trial used an unconventional intermittent dosing schedule, based on the team’s earlier experiments, which resulted in the combination being effective but also tolerable in terms of side effects.
Low-grade ovarian cancer is less responsive to chemotherapy due to its slow growth. It also has fewer genetic mutations, making it less reliant on the cellular pathways that chemotherapy typically targets. The clinical trial showed a reduction in tumour size and halted progression for more than 18 months, demonstrating a better response than chemotherapy in this ovarian cancer type. Later phase trials are now ongoing and have been led by Professor Susanna Banerjee. These trials have been designed to formally show a difference between current standard of care treatments and the new avutometinib and defactinib combination, which is a requirement if regulatory authorities decide to license a drug or drug combination therapy.
Professor Banerji says: “Effective combination therapy should by synergistic – the sum of the effectiveness of individual drugs should be greater than each individual drug’s efficacy. In this case defactinib on its own has very little activity but significantly increases the activity of avutometinib. Combining such drugs is challenging due to side effects such as fatigue, gastrointestinal issues or liver toxicity. Our novel intermittent dosing allowed us to hit the sweet spot between effectiveness and side effects.”
Innovative approaches to cancer treatment
Professor Banerji has a particular interest in overcoming drug resistance, and he is currently pioneering groundbreaking approaches such as evolutionary steering, which could transform how we approach the treatment of cancer, including ovarian cancer.
Evolutionary steering
Evolutionary steering is a unique approach that works to predict and influence how cancer cells will adapt. Using mathematical models and genetic insights, Professor Banerji and Professor Trevor Graham, Director of the Centre for Evolution and Cancer at the ICR, are trying to anticipate the path cancer cells might take in response to treatment.
By using evolutionary steering, researchers at the ICR aim to guide cancer cells into patterns that make them more vulnerable to subsequent treatments, essentially “herding” the cancer cells to a place where they are easier to treat. This process involves manipulating the tumour environment to favour cells that are less resistant to current treatments and maintaining a population of treatment-sensitive cells to supress treatment-resistant ones. These approaches may in the future, transform how we treat ovarian cancer.
Professor Graham says: “The biggest challenge, but also the biggest impact, would be if we can use the information obtained about evolutionary steering and other innovative approaches to design clinical trials and change practice. As we understand more, we might be able to rethink how we currently give chemotherapy, for example giving treatments sequentially rather than in combination. It does go against our current practice, so that's why it’s essential we get more evidence before we can think of using this in the clinic.”
Professor Banerji added: “By effectively playing chess with cancer cells, we can anticipate their moves and stay one step ahead. Ultimately, if we can predict cancer’s next move, we can beat it.”
-organoids.jpg?sfvrsn=edb65d57_1)
Image: Patient-derived High-Grade Serous Ovarian Carcinoma (HGSOC) organoids grown, fixed and imaged in ICR laboratories. Cells were grown and taken from a liquid biopsy (ascites).
Funding challenges in ovarian cancer research
As ovarian cancer is commonly diagnosed at a later stage, curative treatment becomes more difficult due to the cancer becoming advanced. This emphasises the urgent need for new treatments or utilising existing therapies in a smarter way to improve outcomes for patients.
Professor Banerji’s work at the ICR is helping to shift this narrative. His promising work on idetrexed, NXP800 and the combination of avutometinib and defactinib, alongside his collaborations with Professor Graham into evolutionary steering, provides hope that we can transform the landscape of ovarian cancer treatment.
However, the relatively low level of funding for ovarian cancer is making it harder for researchers to carry out the innovative work needed to make new treatments available.
Professor Banerji says: "In the financial year 2020/21, the UK National Cancer Research Institute’s database shows that £10.3m was spent on ovarian cancer compared to £51.5m, £39.5m and £37.6m on breast, colorectal and lung cancer respectively."
Together, we can make a difference
Professor Banerji says: “My generation of researchers stands on the shoulders of previous oncology research giants at the ICR, who pioneered paradigm-shifting drugs – such as carboplatin, the biological discoveries of the BRCA2 gene and the concept of synthetic lethality, which guided how we use PARP inhibitor drugs like olaparib.
“There are often entire laboratories and clinical teams that make stepwise changes in cancer therapy. At the ICR we have a wide range of teams focused on biology, drug discovery, radiotherapy, imaging and clinical trials working on ovarian cancer. In collaboration with our partners at The Royal Marsden, we make a formidable team of committed researchers in different disciplines, all very passionate about our work.
“Supporting ovarian cancer research is essential to fuelling the discoveries that could lead to better therapies and, ultimately, improved outcomes for women worldwide.”
By drawing attention to the unique demands in ovarian cancer research, we aim to secure the crucial resources required to drive the next breakthroughs in ovarian cancer treatment.
With your support, we can ensure that ovarian cancer research is funded at a level that truly reflects its impact on the lives of women and their families around the world.
By advancing the groundbreaking work carried out by Professor Banerji and his colleagues, we can work towards a future where ovarian cancer is no longer a stealthy killer but a disease that we can understand, manage, and even overcome.
Our research is helping more women survive cancer. Please donate today and support the vital work our scientists are doing to defeat cancer.

 .
.