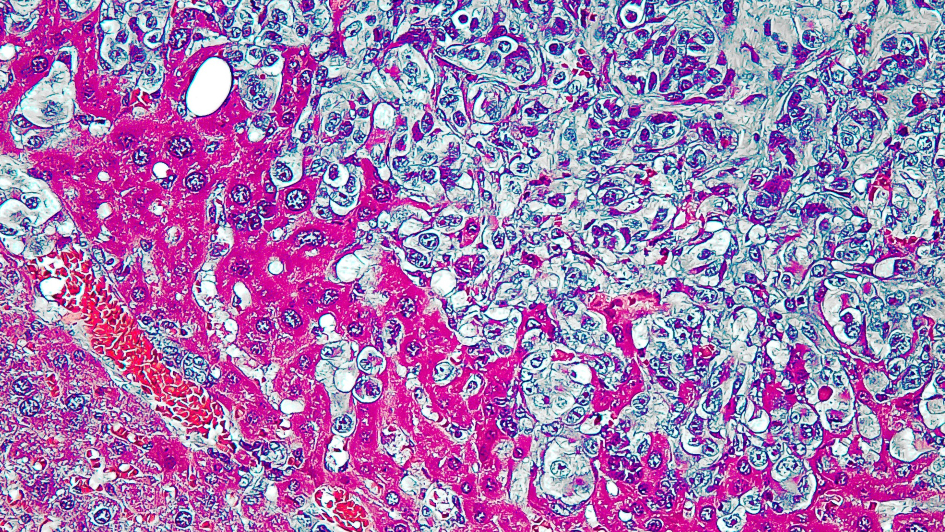
Image: Metastasis in a Kaposi sarcoma mouse model. Credit: Anthony B. Eason via NIH Image Gallery on flickr, CC BY-NC 2.0
Researchers at The Institute of Cancer Research, London, and The Royal Marsden NHS Foundation Trust, working alongside collaborators from around the globe, are set to begin work on the world’s largest digital hub of sarcoma clinical and research data after receiving a £2.5m grant.
Dr Robin Jones at The Royal Marsden NHS Foundation Trust and the ICR will lead the five-year project aiming to produce the digital hub, as well as 3D cell and animal models of sarcoma, that will help predict and test patient response to drugs for high-risk sarcomas using artificial intelligence.
Co-investigators on the project include teams based at based at University of Birmingham, NHS Greater Glasgow and Clyde, The Queen Elizabeth Hospital, University of Edinburgh, Fondazione IRCCS – Istituto Nazionale dei Tumori – Milano, and Instituto de Biomedicina de Sevilla.
The grant is part of a total of £27.4 million being invested by Cancer Research UK and its European partners, Fondazione AIRC and Asociación Española Contra el Cáncer (AECC), through the Accelerator Awards scheme.
Supercharging sarcoma research
Patients with sarcoma have poor survival overall, with 50 per cent seeing their disease spread to other organs, at which point it is incurable. Clinicians have had limited success with a ‘one-size-fits-all’ approach using chemotherapy, and more personalised treatments are needed.
The team hopes to identify new biological markers, or biomarkers, that can be measured and indicate how well a treatment is working for a sarcoma patient.
This will allow clinicians to separate patients into different groups – a process called patient stratification – based on how likely they are to respond to different types of treatment.
The team also hope to develop digital tools and mobile apps that will help facilitate prognosis and treatment, allowing clinicians to better individualise treatment for patients.
Aiming for a step-change in sarcoma treatment
Dr Paul Huang, who leads the Molecular and Systems Oncology Team at the ICR and is Deputy Director of the Joint Sarcoma Research Centre at the ICR and The Royal Marsden, said:
“We are thrilled to be leading this project alongside talented teams from around the world. Most forms of sarcoma are extremely rare, which can make it harder to research. But these cancers are devastating for patients and their families.
“Progress in finding new treatments for sarcoma has stalled – over the past two decades there have been no clinical trials in the pre-operative setting led by the pharmaceutical industry. This investment will allow us to take an integrated, multidisciplinary approach, and hopefully will lead to a real step-change in sarcoma treatment.”
We have a proven track-record of awe-inspiring research, which is transforming the lives of cancer patients around the world. This work is made possible by an extraordinary community of generous donors, which includes individuals, trusts and foundations and charity partners.
The patient perspective
Bill Russell, aged 70 from Aylesbury in Buckinghamshire, is living with vascular sarcoma, one of the high-risk sarcomas that this research will focus on. He is hopeful about where this funding will take sarcoma research:
“There are not many of us – people living with sarcoma – and because sarcoma occurs in many different organs and in different ways, there is a need to collect lots of information from around the world.
“We are all different, so a more personalised treatment may work better and mean fewer side effects for us.
“I hope that this project will pull together lots of information and knowledge that can be used to improve the diagnosis and treatment of sarcomas, so that people like me have more hope for the future.”
Zooming in on cancer cells
Professor Chris Bakal, who leads the Dynamical Cell Systems team at the ICR, will also benefit from the Accelerator Awards programme.
Bio-engineering work in recent years has improved how researchers can model tumours in the lab, but imaging these models continues to present a challenge.
Together with a team led by Professor Paul French at Imperial College London, Professor Bakal’s team will work on 3D models of cancer in the lab, and develop imaging techniques which will allow them to examine the mini-tumours down to the level of a single cell.
A global effort to tackle cancer
Commenting on the new funding, Dr Iain Foulkes, executive director of research and innovation at Cancer Research UK, said:“If current trends continue, the world will see a 60% increase in cancer cases over the next two decades. Cancer is a global problem and no one country can tackle it alone.
“Now the UK has left the European Union, the need to retain collaborative cancer research between the EU and the UK has never been greater. This partnership will also strengthen UK cancer research by the sharing of expertise, new technologies and research talent.”

 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
 .
.
.jpg?sfvrsn=faf78b2_4) .
.
 .
.
 .
.
 .
.