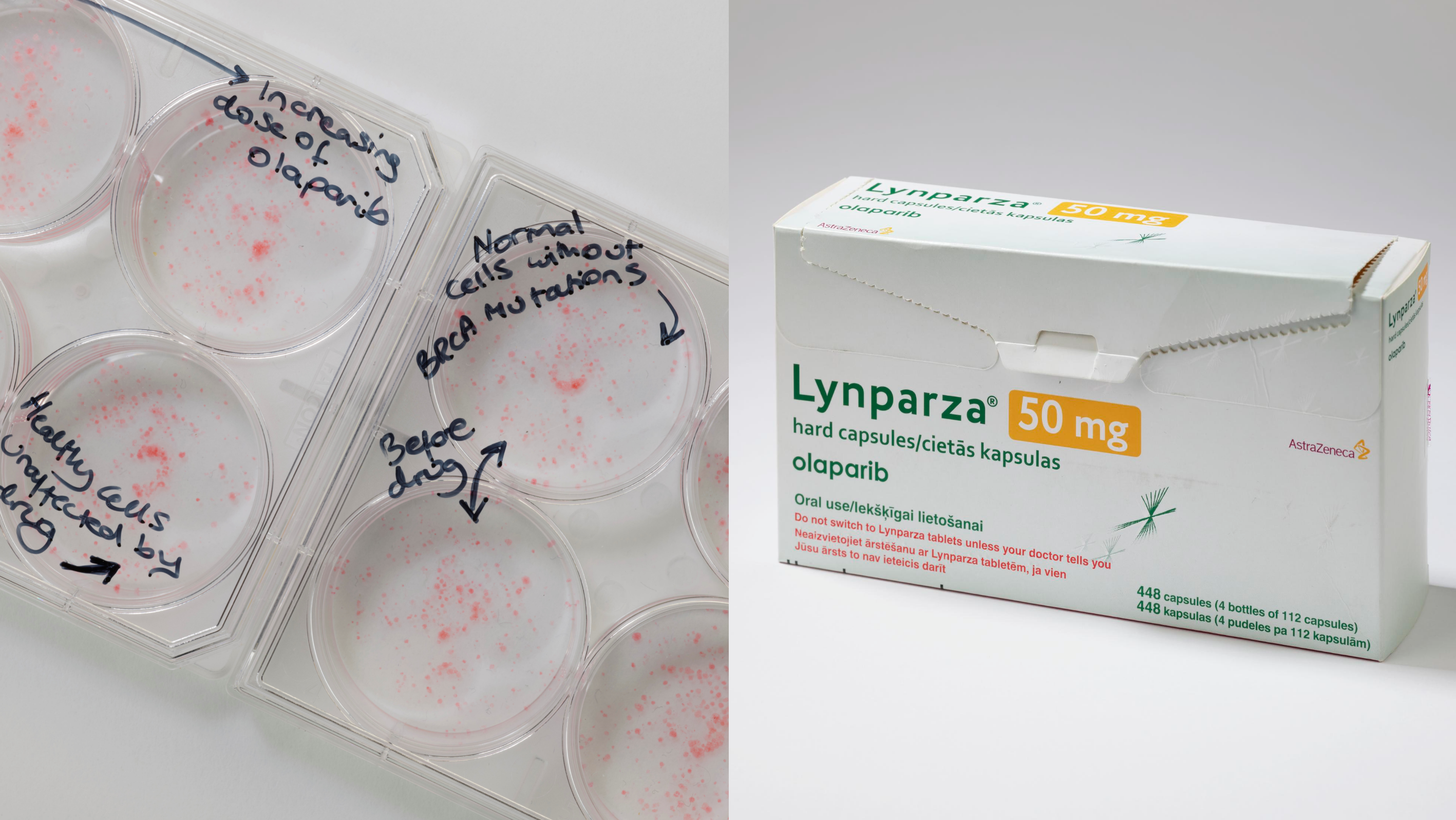
CHHiP
Conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer.
Disease site: Urological cancers Prostate cancer
Treatment Modality: Radiotherapy
Status: In follow-up
Trial details
CHHiP is a phase III, multicentre, randomised controlled trial to see whether hypofractionated radiotherapy schedules (fewer fractions in higher doses) for localised prostate cancer could improve the therapeutic ratio by either improving tumour control or reducing normal tissue side effects.
A total of 3,163 participants were recruited to CHHiP over nine years. Patients received one of three radiotherapy schedules:
- Conventional radiotherapy fractionation using a total dose of 74 Gy in 37 fractions over 7.4 weeks, using conformal and intensity modulated radiotherapy techniques
- Hypofractionation of 57 Gy in 19 fractions over 3.8 weeks
- Hypofractionation of 60 Gy in 20 fractions over 4 weeks
Participants will be followed up for 15 years.
To learn and understand more about your diagnosis, and the care and treatment you receive, we have asked your permission to access information in your NHS medical records about your health conditions, treatment, progress, and (in the event of your passing away for whatever reason) mortality information. To do this your NHS number and date of birth was securely shared by the NHS digital National Cancer Registration and Analysis Service (NCRAS) to allow your records to be located. Data supplied by NCRAS will relate to your cancer and care over time, and will be linked to the information obtained from your hospital as part of the trial. All the information shared with NCRAS will be governed by strict rules covering data protection and confidentiality. You can read more about NCRAS: https://www.ndrs.nhs.uk. You can find out more about how we will protect your information here: https://www.icr.ac.uk/legal/privacy/research-privacy-notice.
Results
The five-year outcomes of CHHiP were first presented at the NCRI Cancer Conference 2015 and published in Lancet Oncology in June 2016. The ICR prepared a press release to explore these results in more detail.
After following patients up for five years, analysis showed that hypofractionated radiotherapy of 60 Gy in 20 fractions was found to be non-inferior to conventional radiotherapy fractionation for prostate cancer progression, and not associated with significant changes in late toxicity.
In addition to potentially saving each patient 17 hospital visits for their prostate radiotherapy, 60 Gy in 20 fractions appears effective and safe, and may be recommended as a new standard of care.
We have prepared a plain English summary of the CHHiP results at 5 years, which can be viewed and downloaded here.
Further information
Chief Investigator: Professor David Dearnaley, The Royal Marsden NHS Foundation Trust
ICR-CTSU Scientific Lead: Dr Emma Hall
Trial management contact: [email protected]
ISRCTN: 97182923
Sponsor: The Institute of Cancer Research
Funding: Cancer Research UK (CRUK/06/016)
View CHHiP on the National Institute for Health Research website: NIHR - Be Part Of Research
A plain English summary is available from Cancer Research UK.
Publications and presentations
Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, Graham J, Kirkbride P, Logue J, Malik Z, Money-Kyrle J, O'Sullivan JM, Panades M, Parker P, Patterson H, Scrase C, Staffurth J, Stockdale A, Tremlett J, Bidmead M, Mayles H, Naismith O, South C, Gao A, Cruickshank C, Hassan S, Pugh J, Griffin C, Hall E; on behalf of the CHHiP Investigators. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016; Published online ahead of print; doi: 10.1016/S1470-2045(16)30102-4.
Ahmed M, Dorling L, Kerns S, Fachal L, Elliott R, Partliament M, Rosenstein BS, Vega A, Gómez-Caamaño A, Barnett G, Dearnaley DP, Hall E, Sydes M, Burnet N, Pharoah PD, Eeles R, West CM. Common genetic variation associated with increased susceptibility to prostate cancer does not increase risk of radiotherapy toxicity. Br J Cancer. 2016; 114(10): 1165-74.
Wilkins A, Mossop H, Syndikus I, Khoo V, Bloomfield D, Parker C, Logue J, Scrase C, Patterson H, Birtle A, Staffurth J, Malik Z, Panades M, Eswar C, Graham J, Russell M, Kirkbride P, O'Sullivan JM, Gao A, Cruickshank C, Griffin C, Dearnaley D, Hall E. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015; 16(16): 1605-16.
Hall E, Syndikus I, Mossop H, Staffurth J, Scrase C, Panades M, Money-Kyrle J, Logue J, Khoo V, Graham J, Bloomfield D, Birtle A, Tremlett J, Naismith O, Mayles H, Hassan S, Cruickshank C, Griffin C, Dearnaley D. 5 year outcomes of a phase III randomised trial of conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer [CRUK/06/016]: report from the CHHiP Trial Management Group. Presented at: National Cancer Research Institute Cancer Conference 2016; 2016 Nov; Liverpool, UK.
Naismith O, Griffin C, Syndikus I, Mayles H, Hall E, Dearnaley D; CHHiP Trial Management Group. Forward and inverse-planned intensity-modulated radiotherapy (IMRT) in the CHHiP trial: a comparison of dosimetry and normal tissue toxicity. J Clin Oncol. 2014; 32(Suppl 4); 37.
Dearnaley D, Griffin C, Syndikus I, Scrase C, Thomas S, Naismith O, Mayles P, Staffurth J, Hall E; on behalf of the CHHiP Trial Management Group. IGRT for prostate cancer - results from the CHHiP IGRT Phase II sub-study. Radiother Oncol. 2014; 111(Suppl 1): 70 #OC-155.
Dearnaley D, Syndikus I, Sumo G, Bidmead M, Bloomfield D, Clark C, Gao A, Hassan S, Horwich A, Huddart R, Khoo V, Kirkbride P, Mayles H, Mayles P, Naismith O, Parker C, Patterson H, Russell M, Scrase C, South C, Staffurth J, Hall E. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012; 13(1): 43-54.
Dearnaley D, Naismith O, Sumo G; on behalf of the CHHiP Trial Management Group. A dosimetry comparison of forward- and inverse-planned IMRT for prostate cancer in the CHHiP trial. Radiother. Oncol. 2011; 99(Suppl 1): S585 #1573.
Naismith O, Dearnaley D, Hall E; on behalf of the CHHiP Trial Management Group. A survey of the benefits of RT processes and techniques of participating in the CHHiP trial. Radiother. Oncol. 2011; 99(Suppl 1): S585 #1575.
Dearnaley DP, Sumo G, Syndikus I, Khoo V, Patterson H, South C, Mayles P, Mayles H, Gao A, Hall E; on behalf of the CHHiP Trial Management Group. Initial toxicity results of the Phase III conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer (CHHiP) trial. Presented at: ASCO 2009 Genitourinary Cancers Symposium; 2009 Feb; Orlando, FL, USA.
Dearnaley D, Syndikus I, Norman A, Khoo V, South C, Naismith O, Mayles W, Huddart R, Horwich A, Parker C. Conventional or hypofractionated high dose intensity modulated radiotherapy in prostate cancer: Preliminary report on acute and late toxicity (ISRCTN97182923). A Phase III multicentre trial (CHHiP). Presented at: ASCO Prostate Cancer Symposium; 2007 Feb; Orlando, FL, USA.
Naismith OF, Clark CH, Mayles HM, Moore AR, Bidmead AM, Dearnaley DP; on behalf of the CHHiP trial collaborators. Quality Assurance of dosimetry in centres participating in the CHHiP prostate radiotherapy trial. Clin. Oncol. (R. Coll. Radiol). 2007; 19(Suppl 3): S14 #28.
South C, Clark CH, Norman A, Gao A, Dearnaley D. CHHIP vs RTO1: a comparison of dose distributions. Clin Oncol (R Coll.Radiol.) 2005; 17(2): S32.
New drug hope for prostate cancer patients