Intercontinental
Intercontinental is an international phase III randomised trial comparing intermittent versus continuous androgen suppression for patients with prostate-specific-antigen progression in the clinical absence of distant metastases following radiotherapy for prostate cancer.
Disease site: Urological cancers Prostate cancer
Treatment Modality:
Status: Closed
Trial details
The aim is to evaluate the use of intermittent androgen suppression (IAS) for overall survival, the potential for an improved quality of life during the non-treatment interval and decreased cost of therapy compared to continuous androgen ablation.
The trial is coordinated by the National Cancer Institute of Canada with ICR-CTSU responsible for UK centres. In the UK the trial is supported by the NCRI Prostate Clinical Studies Group.
The trial has recruited 1,386 patients internationally.
Chief Investigator: Professor David Dearnaley, The Royal Marsden NHS Foundation Trust
ICR-CTSU Scientific Lead: Dr Emma Hall
Trial management contact: Sarah Kernaghan - Clinical Trials Programme Manager, [email protected]
ISRCTN: 22761545
Sponsor: The Institute of Cancer Research
Funding: Cancer Research UK (CRUKE/01/013)
Further information
Further information on the Intercontinental trial may be found on the following sites:
Patient friendly information on the Intercontinental trial at CancerHelp UK
Publications and presentations
Hall E, Patel S, Clarke N, Dearnaley D. A phase III randomized trial of post-radiotherapy intermittent androgen suppression (IAS) for prostate cancer patients with rising PSA-survey of interest. Clin. Oncol. (R. Coll. Radiol). 2002;14:S48 #D1.1
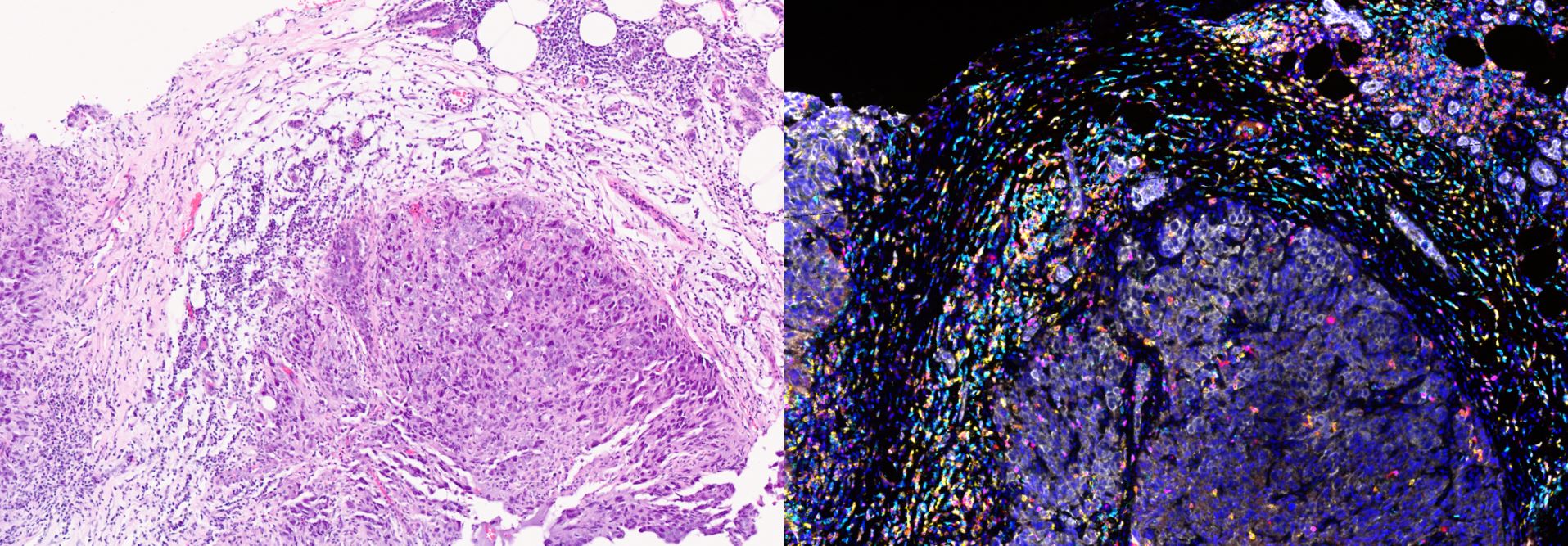
Neoadjuvant radiotherapy provides unique insights into breast tumour immune microenvironment