HERA
A randomised three arm multicentre comparison of 1 year and 2 years of trastuzumab (Herceptin®) versus no trastuzumab in women with HER2+ primary breast cancer who have completed adjuvant chemotherapy.
Disease site: Breast cancer
Treatment Modality:
Status: Closed
Trial details
The primary aim of the study is to compare disease-free survival (DFS) in patients with HER2 over expressing primary breast cancer randomised to Herceptin for 1 year or no further therapy or to Herceptin for 2 years or no further therapy.
The trial recruited 5,102 women with primary breast cancer that overexpresses HER2 (determined by IHC 3+ or FISH positive). Following the positive results at the interim analysis, all HERA patients on the observation arm have been offered the chance of receiving Herceptin treatment either for 1 year by direct assignment or by randomisation to either 1 or 2 years treatment, or they can choose to remain on the observation arm.
This is an international study run under the auspices of the Breast International Group (BIG).
There are five collaborative groups within the UK which participate in the study:
- National Cancer Research Institute Breast Clinical Studies Group (NCRI BCSG)
- Anglo Celtic Cooperative Oncology Group (ACCOG)
- Yorkshire Breast Cancer Research Group (YBCRG)
- European Organisation for Research and Treatment of Cancer (EORTC)
- International Collaborative Cancer Group (ICCG)
UK Chief Investigator: Professor I. E. Smith, Royal Marsden Hospital, Sutton
ISRCTN: 82811952
Sponsor: Roche
Funding: Roche
Further information
More information about the trial can be found on Roche's HERA webpage or see the BIG HERA webpage for a list of publications arising from the research.
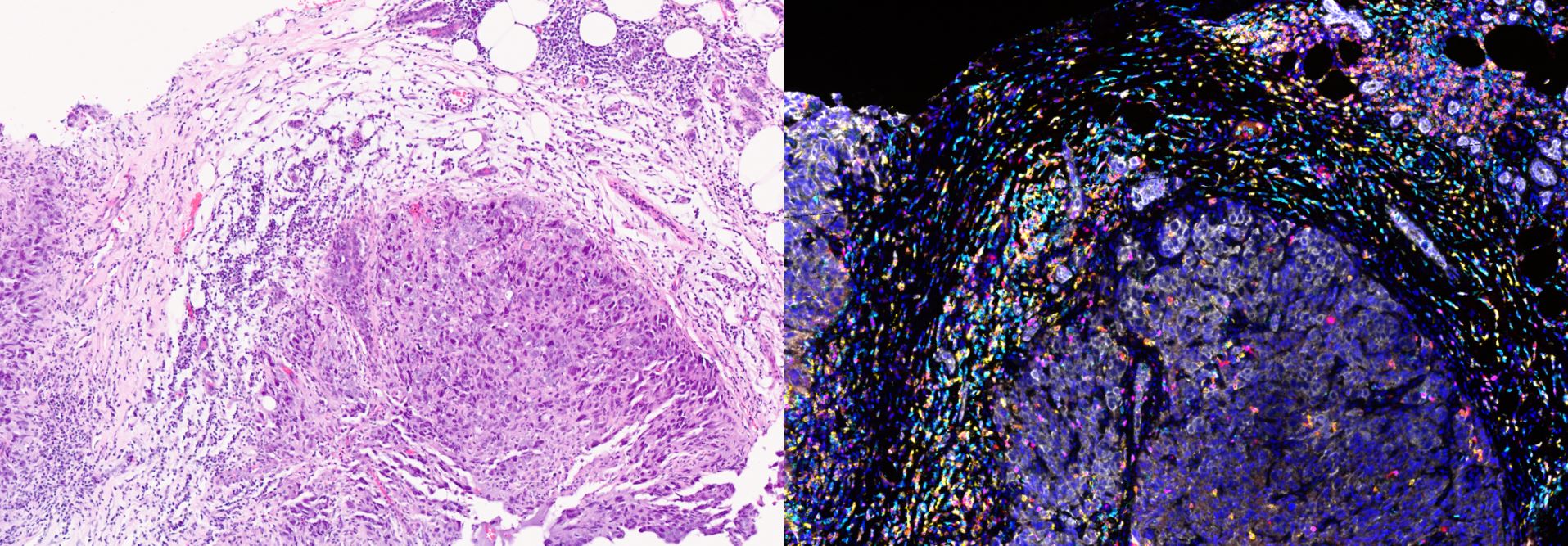
Neoadjuvant radiotherapy provides unique insights into breast tumour immune microenvironment