COSTAR
A multicentre randomised study of cochlear sparing intensity modulated radiotherapy versus conventional radiotherapy in patients with parotid tumours.
Disease site: Head and neck cancer
Treatment Modality:
Status: Closed
Trial details
COSTAR is a phase III multi-centre randomised controlled trial designed to demonstrate a difference in the proportion of patients suffering sensori-neural hearing loss of at least 10dB in bone conduction at 4000 Hz, as assessed by an audiogram done 12 months post radiotherapy, in patients treated with cochlea-sparing IMRT and conventional radiotherapy.
The work is supported by the NCRI CTrad Group and the NCRI Head and Neck Cancer Studies Group.
Chief Investigator: Dr Chris Nutting, The Royal Marsden NHS Foundation Trust
ICR-CTSU Scientific Lead: Dr Emma Hall
Trial management contact: Mark Sydenham - Trial Manager, [email protected]
ISRCTN: 81772291
Sponsor: The Royal Marsden NHS Foundation Trust
Funding: Cancer Research UK (CRUK/08/004)
Further information
Patient friendly information on the COSTAR trial at Cancer Research UK
Publications and Presentations
Nutting CM, Morden JP, Beasley M, Bhide S, Cook A, De Winton E, Emson M, Evans M, Fresco L, Gollins S, Gujral D, Harrington K, Joseph M, Lemon C, Luxon L, van den Blink Q, Mendes R, Miah A, Newbold K, Prestwich R, Robinson M, Sanghera P, Simpson J, Sivaramalingam M, Srihari NN, Sydenham M, Wells E, Witts S, Hall E; COSTAR Investigators.
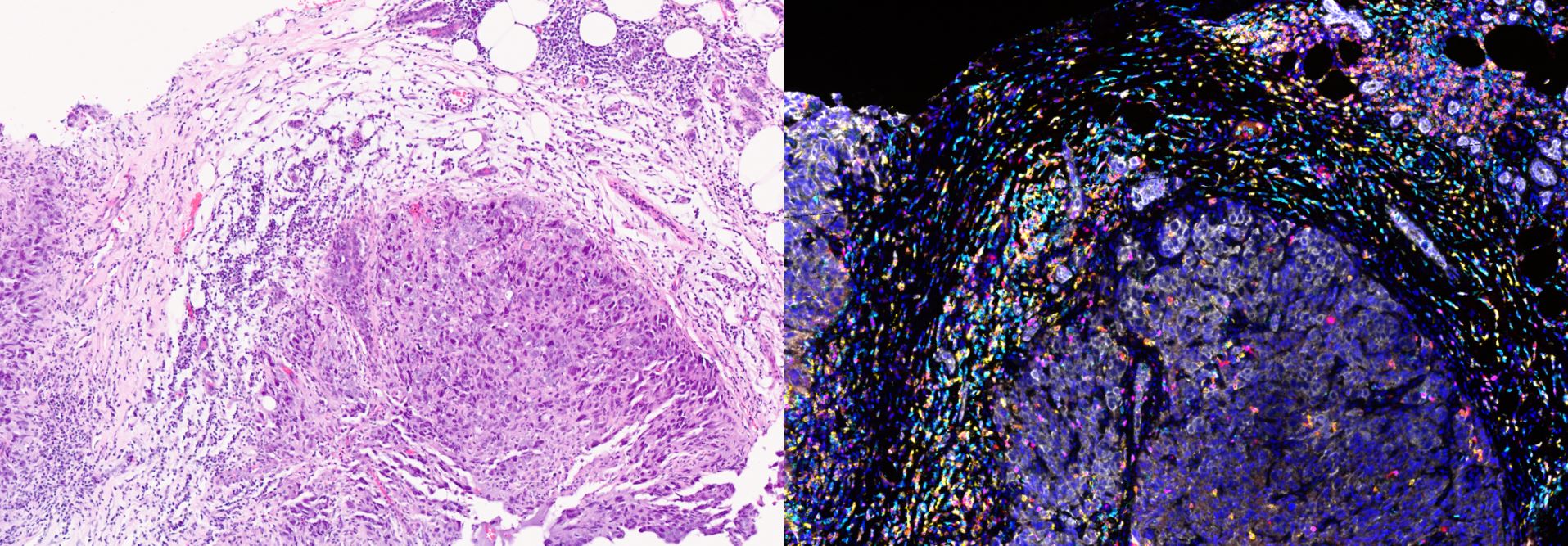
Neoadjuvant radiotherapy provides unique insights into breast tumour immune microenvironment