AZURE
Does adjuvant zoledronic acid reduce recurrence in patients with high risk localised breast cancer?
Disease site: Breast cancer
Treatment Modality:
Status: Closed
Trial details
AZURE is a prospective, randomised, open label, parallel group trial to determine whether adjuvant treatment with zoledronic acid with (neo)adjuvant chemotherapy and/or (neo)adjuvant endocrine therapy is superior to (neo)adjuvant chemotherapy and/or (neo)adjuvant endocrine therapy alone in improving disease free and bone metastasis free survival in women with Stage II/III breast cancer.
This international trial opened to recruitment in September 2003, and closed to recruitment in January 2006 with over 3,300 women randomised.
AZURE is co-ordinated centrally by the Clinical Trials Research Unit at the University of Leeds. ICR-CTSU acted as a local trials office for 25 sites (600 patients) during the interventional phase of the trial.
Further information
Chief Investigator: Professor Robert Coleman, Sheffield
ISRCTN: 79831382
Sponsor: University of Sheffield
View AZURE on the National Institute for Health Research website: NIHR - Be Part Of Research
Patient friendly information is available from the following link:
Publications and presentations
Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, Gil M, Houston SJ, Grieve RJ, Barrett-Lee PJ, Ritchie D, Pugh J, Gaunt C, Rea U, Peterson J, Davies C, Hiley V, Gregory W, Bell R; AZURE Investigators. Breast cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011; 365(15): 1396-405.
Coleman R, Woodward E, Brown J, Cameron D, Bell R, Dodwell D, Keane M, Gil M, Davies C, Burkinshaw R, Houston SJ, Grieve RJ, Barrett-Lee PJ, Thorpe H. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01-04) for women with stage II/III breast cancer. Breast Cancer Res Treat. 2011; 127(2): 429-38.
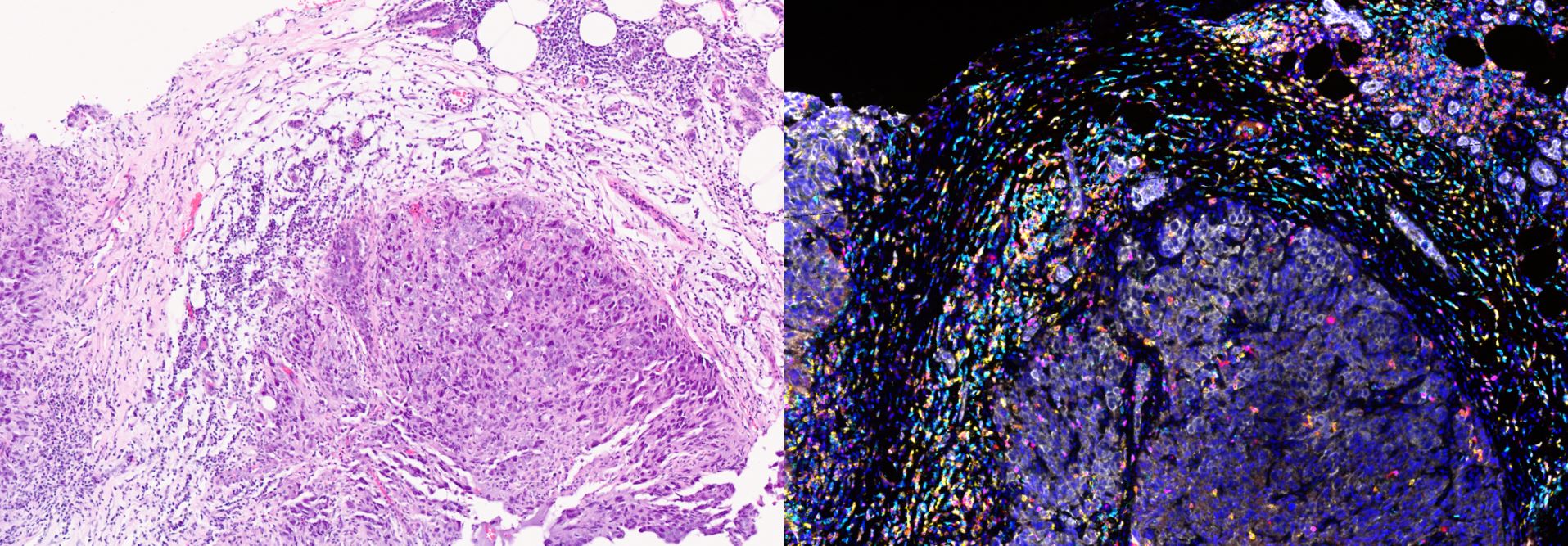
Neoadjuvant radiotherapy provides unique insights into breast tumour immune microenvironment