ICCG HMFEC Trial
FE 50 C versus FE 75 C with or without sequential hormone therapy in women with node positive premenopausal breast cancer.
Disease site: Breast cancer
Treatment Modality:
Status: Closed
Trial details
International multicentre randomised study of adjuvant chemotherapy comparing efficacy and toxicity of ‘standard’ dose 5-fluorouracil, epirubicin and cyclophosphamide (FE 50 C) with a ‘higher’ dose 5-flouorouracil, epirubicin and cyclophosphamide (FE 75 C) and evaluating the additional benefit of sequential hormone therapy. 785 premenopausal women with node positive primary breast cancer from 7 countries were randomised to receive 8 cycles of FE 50 C or FE 75 C given at 3 weekly intervals with or without sequential hormone therapy. Depending on hormonal status at the end of chemotherapy patients randomised sequential therapy were prescribed either GnRH agonist for 3 years or tamoxifen for 5 years.
This is an International Collaborative Cancer Group (ICCG) trial (C/9/91).
Publications and presentations
Bliss J, Wils J, Marty M, Coombes G, Lawrence D, Coombes RC, on behalf of the ICCG. Evaluation of the tolerability of FE50C versus FE75C in a prospective randomised trial in adjuvant breast cancer patients. J. Clin. Oncol. 2002;20:15S #2017
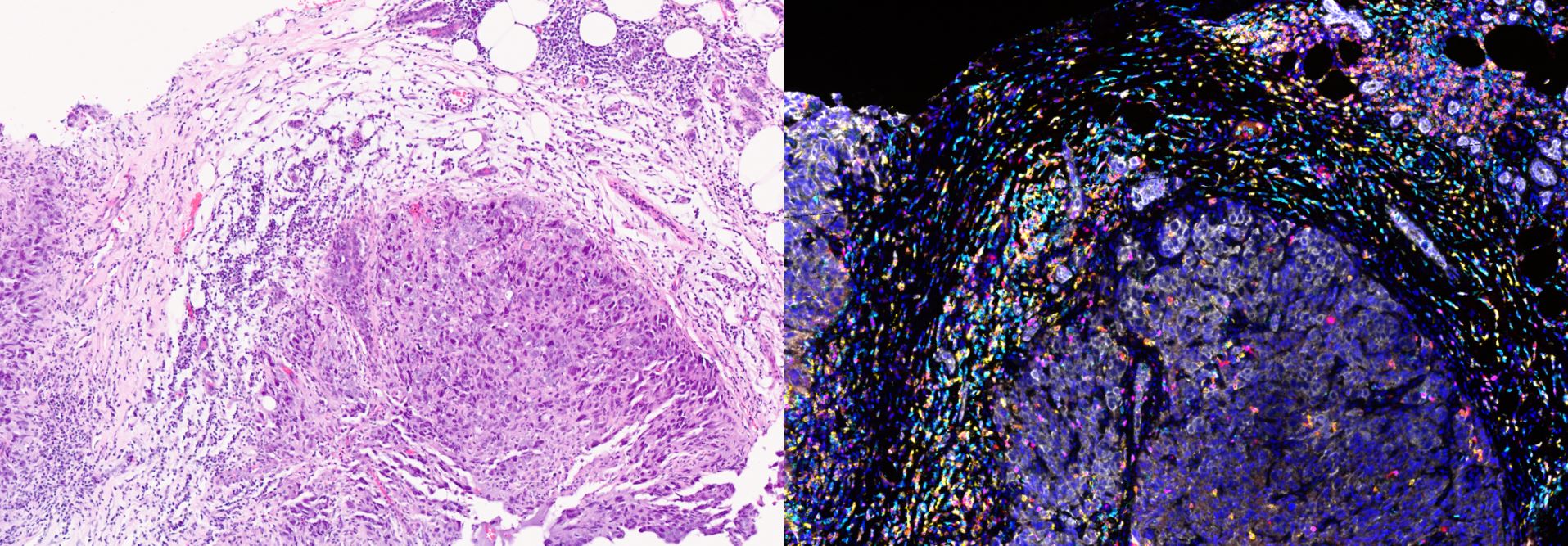
Neoadjuvant radiotherapy provides unique insights into breast tumour immune microenvironment