Professor Paul Workman was 37 and already well established as a cancer researcher when his mother, Ena, died aged 68 from a rare bone tumour known as chordoma. About one in a million people are affected by the condition, for which there are no targeted drug treatments.
“I was frustrated to hell that I couldn’t do a damned thing,” said Paul, who later became head of the ICR’s Centre for Cancer Drug Discovery (CCDD) and then chief executive of the ICR. He led the Centre for 18 years and ICR for seven years, both with great success, before stepping down as chief executive in 2021 – but remains at the ICR as Harrap Professor of Pharmacology and Therapeutics.
“Thirty-six years ago, there was nothing we could do to treat chordoma beyond radiotherapy and surgery. There was little understanding of the disease and no approved drugs were available to help my mother. It was really painful at the time, and it has absolutely motivated me ever since.”
Discovery of the target
That grim state of affairs could soon be about to change. Paul and his colleagues, working as part of an international collaboration, recently pinpointed a key protein, known as brachyury, which they realised was crucial to the growth of chordoma cancer cells in a patient’s body.
“I was invited to join the team due to my decades of experience in cancer drug discovery. It was a complete coincidence that my collaborators were studying the cancer I was determined to tackle – one of those wonderful things that sometimes fall into one’s lap.”
The discovery of brachyury’s role caused great excitement among researchers because it suggested a route for attacking chordoma: block the protein brachyury and this would arrest the cancer cells whose growth it was promoting. All that was needed was a drug that could effectively attack the protein.
Finding a way to drug the target
The problem was the challenging structure of brachyury which, as a transcription factor responsible for controlling gene activity, was considered to be ‘undruggable’. “Think of it like a rock climber scaling a cliff. You need crevices to get your toes and fingers into – but the surface of the brachyury protein seemed very smooth. It was a tough nut for us to crack.”
However, in a paper published in Nature Communications last week, Paul – with colleagues in the University of Oxford and University of North Carolina – revealed that, after determining the 3D structure of brachyury in unsurpassed detail, they had pinpointed several sites on its surface which could be used as targets for specially designed drugs.
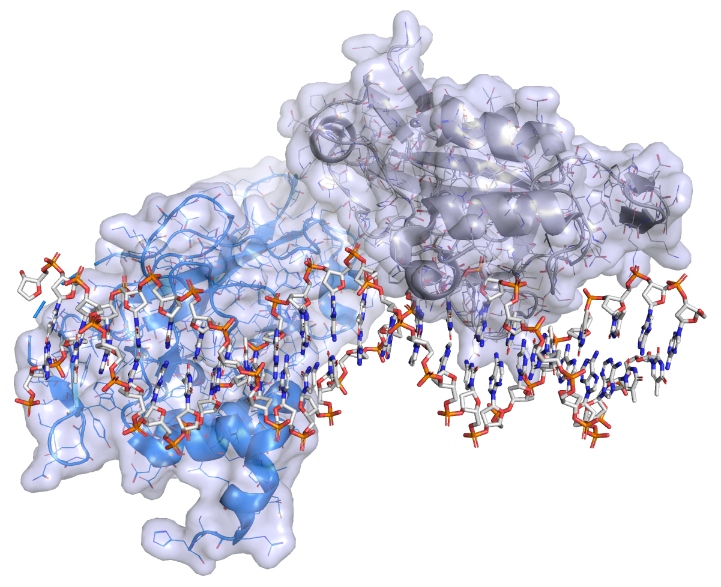
The brachyury protein bound to DNA. Credit: Paul Workman, ICR
A breakthrough using advanced technologies
This breakthrough was achieved by using X-ray crystallography at one of the world’s most powerful generators of X-rays: the Diamond Light Source synchrotron in Didcot, Oxfordshire.
The progress towards the discovery of drugs to tackle chordoma has taken many years and involved other advanced technologies. In particular, a technique known as crystallographic fragment screening, also carried out using Diamond Light Source, played a key role in highlighting sites where potential drugs could best latch on to the brachyury protein. “This has allowed the team to develop the best-fitting prototype drugs that can fasten on to the protein’s surface,” said Paul.
As a result of this work, the team have already been able to design several promising compounds – based on the initial tiny chemical fragments that they found to bind – that are now being used to create potential treatments that could attack brachyury and inhibit or even destroy the protein. In this way, doctors may soon be able to tackle chordoma, a condition that has until now resisted efforts to find drugs that combat its growth and spread.
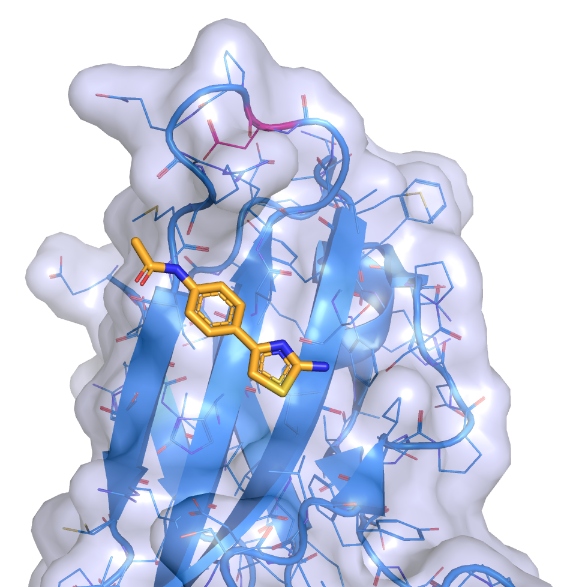
A small molecule ligand (drug precursor) binding in a shallow groove on the surface of brachyury. Credit: Paul Workman, ICR
“The reality that I could really do something useful against the disease that killed my mother is really quite thrilling. It has taken considerable effort by a lot of excellent scientists from centres on both sides of the Atlantic, but it has been worth it.”
Co-opting the body’s natural waste disposal system
Crucially, the compounds and techniques that were developed in this work to tackle brachyury and chordoma could be used to improve treatments for other, more common, cancers. “Firstly, brachyury appears to be involved in the metastatic spread of other tumours, which means that future drugs that block its activities could also help to obstruct the spread of other cancers. In addition, the technique that we have used to work out how to target brachyury could be applied to other ‘undruggable’ proteins – which are responsible for a very large number of other cancers.”
Paul says the greatest potential to achieve a clinical drug for brachyury lies with a system called targeted protein degradation – something the ICR is advancing through its Centre for Protein Degradation. This involves a process of co-opting a cell’s natural waste disposal system to remove the offending protein.
“One part of the drug will bind to the target protein, while the other part engages directly with the cells’ natural protein destruction machinery, which then degrades the protein, thereby removing it from the chordoma cells. So, we will use the body’s own waste disposal system to deal with brachyury.”
However, Paul stressed that more research was still needed to perfect a drug that would be effective in treating chordoma. “This initial work represents a real breakthrough in solving the 3D structure of human brachyury and discovering multiple small molecules that bind to crevices in its surface. Now, we need to make them bind tighter and potentially turn them into brachyury degraders. Then we’ll evaluate them in chordoma cell lines and subsequently in chordoma models in animals, before we start clinical trials in humans. That could take five years to complete. Then, hopefully, we will finally be ready to tackle the challenge of chordoma in patients.”
Late diagnosis of chordoma
Paul is the only child of John and Thomasena (Ena) Workman. His father worked in the steel industry, while his mother was active in various community projects in their home town, Workington, in Cumbria. His father died of bowel cancer whilst he was completing his PhD, many years before his mother succumbed to chordoma.
Ena Workman’s diagnosis was complicated by the fact that she had suffered severe back pain for much of her life, after developing tuberculosis during the war, followed by arthritis. This could have led to her diagnosis – in which chordoma began as a tumour at the base of her spine – being missed in its early stages.
Image: Paul as a young boy with his parents
“Brachyury plays a role in the embryo to promote the development of the notochord, a precursor of the spine,” said Paul. In mice, brachyury seems to be involved in the creation of the tail. Around 25 million years ago, the ancestors of humans had a mutation in the brachyury gene which means that we don’t develop tails, and that we walk on two legs. “In humans, brachyury is switched off in the embryo and the notochord disappears after birth. However, in a very few cases remnants of notochord persist in the adult, and when they do it can trigger chordoma driven by brachyury, as was the case with my mother.”
Paul and the team are excited that drugging brachyury could have minimal side effects, as the protein is found so rarely in cells other than those which drive the chordoma cancer.
This discovery has been made possible by the Chordoma Foundation, a charity set up by Josh Sommer, who was himself diagnosed with chordoma in 2006 and was unwilling to accept the limited treatment options available to him. The project also received funding from the Mark Foundation for Cancer Research, and was enabled by the Structural Genomics Consortium.
A notable feature of the research is that it was carried out following the principles of open science. All of the work is made public, including the chemical structures, and the results are not patented. The international team of researchers hope that this will accelerate the discovery of one or more drugs to treat this one-in-a-million cancer, chordoma.Image:
‘I feel very proud’
Professor Paul Workman, Harrap Professor of Pharmacology and Therapeutics at The Institute of Cancer Research, London, said:
“I feel very proud that this breakthrough now has the potential to make a difference to the lives of those, like my mother, who suffer from chordoma. I would also like to pay tribute to the team of fantastic multidisciplinary scientists that made this great progress possible, together with our generous funders and the chordoma patients and families who inspire us every day.”
Professor David Drewry, Principal Investigator at the Structural Genomics Consortium, Member of the UNC Lineberger Comprehensive Cancer Center, and Professor in the UNC Eshelman School of Pharmacy at the University of North Carolina Chapel Hill, said:
“First, I am so grateful for the support we received from the Chordoma Foundation, the Mark Foundation for Cancer Research, and Chordoma patients, and their friends and families. Chordoma patients deserve cutting edge research hunting for ways to halt this disease, and the generous support of these groups and individuals made a tangible difference in our work. I’m thrilled that the first stage of this important project has come to fruition. Team members worked creatively and persistently to get us to this point. Having small molecules that bind to brachyury is an important step that can now launch efforts to discover chemical probes for this protein, and ultimately to identify new medicines to treat chordoma.”
Dr Joseph Newman, Group Leader in DNA repair at the Centre for Medicines Discovery, University of Oxford, said:
"I am grateful for the support from the Chordoma Foundation and Mark Foundation and the engagement from the Chordoma community. We started the structural work on brachyury back in 2017, always releasing our structures immediately to help stimulate brachyury drug discovery efforts within the community. The fragment screening was achieved with the help of the XChem team at Diamond Light Source. It has been fantastic working together with the chemistry team at UNC and the cancer pharmacology team at the ICR to progress the fragment hits into potent ligands and reach this important milestone. I am optimistic about the potential for further progression towards drugs that can ultimately benefit chordoma patients."
‘Major implications for chordoma and other cancers’
Josh Sommer, Executive Director of the Chordoma Foundation, said:
“This work provides a crucial set of building blocks for drugging brachyury, which could have major implications for chordoma and other cancers. And the team's commitment to open data sharing throws open the door to brachyury drug discovery in labs across the world. This work reflects the results of an impressive, industrial-scale early drug discovery campaign prosecuted collaboratively by the Institute of Cancer Research, London, University of North Carolina, and the University of Oxford. We are grateful to the team for their seminal contributions to brachyury drug discovery and are happy to have been able to support their efforts.”
Dr Ryan Schoenfeld, Chief Executive Officer of The Mark Foundation, said:
"The Mark Foundation believes that to make meaningful progress in the fight against cancer, we must challenge conventional wisdom. This team's innovative approach to targeting the 'undruggable' brachyury using cutting-edge chemistry and structural biology exemplifies this approach. Their collaborative work not only opens new treatment avenues for chordoma but also highlights the broader potential of targeting transcription factors across cancer types."
.jpg?sfvrsn=70f78f2b_1)