.jpg)
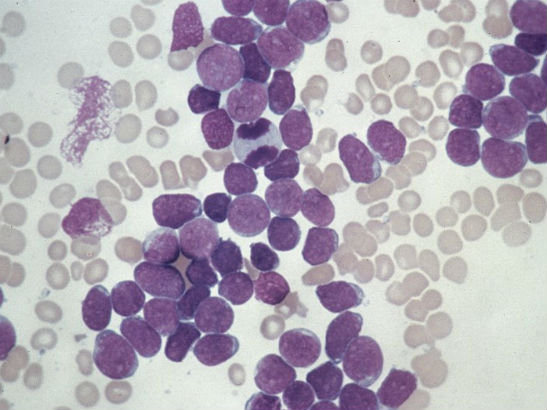
Image: A small, hypolobated megakaryocyte in a bone marrow aspirate, typical of chronic myelogenous leukemia. (Credit: Difu Wu/CC BY-SA 3.0)
The key genetic events responsible for initiating the early stages of a type of childhood leukaemia have been identified by scientists at The Institute of Cancer Research, London.
Scientists have tracked the branching pattern of evolution in a form of childhood leukaemia – and identified a ‘founder’ mutation that may be the trigger for development of the disease. They believe the findings could be used in the development of new targeted drugs.
The research, which was funded by the blood cancer research charity Bloodwise, is published in the journal Leukemia.
'Darwinian' evolution of genetic faults
Leukaemia develops when DNA faults – or ‘mutations’ – occur in maturing blood cells, triggering further genetic mutations that cause the cells to grow out of control.
Genetic faults in childhood leukaemia cells evolve in a ‘Darwinian’ fashion – mutations that make certain cancer cells most resistant to treatment or multiply quickest are more likely to survive and drive the cancer forward.
Cancer cells are constantly evolving genetically, explaining why cancer can sometimes return in a more aggressive form after treatment.
The ICR's Enterprise Unit has an unrivalled track record at discovering new cancer drugs and medical technologies. This has helped ensure that our research delivers maximum benefit for cancer patients.
Identifying 'founder events'
The ICR team used DNA analysis techniques to examine individual leukaemia cells from 19 different children and young adults with STIL-TAL1-positive T-cell acute lymphoblastic leukaemia (T- ALL), in order to determine the sequence in which these faults develop. T-ALL makes up around one in five cases of leukaemia in children and young adults.
The researchers found that certain genetic alterations – the gene fusion STIL-TAL1, as well as inactivation of the CDKN2A gene - occurred very early in leukaemia development.
The fusion between the STIL and TAL1 genes was likely to be a 'founder event' for this type of leukaemia, present in all the cancer cells, meaning that targeting signalling pathways that are affected by this gene fusion could offer an effective way to treat the disease.
The PTEN gene, which is known to play an active role in suppressing cancer, was also deactivated in many individual leukaemia cells. Half of the patients examined had errors affecting the PTEN gene in their cancer cells.
This suggests that, while they occur later than the STIL-TAL1 gene fusion, PTEN gene alterations are key to maintaining leukaemia growth and survival.
More effective and kinder treatments
Dr Caroline Furness, who undertook the research in the laboratory of Professor Mel Greaves at the ICR said:
"We need to understand how cancers evolve and unpick which mutations are key to triggering cancer development, and which are important for driving its growth and spread.
“Our study uncovered these crucial mutations in a type of leukaemia that accounts for around a quarter of cases of T-cell leukaemia in children and young adults.
“This will help us to develop more effective treatments, especially in those children who relapse, and kinder treatments that won’t cause life-long side effects.”
'A need to improve survival further'
Dr Alasdair Rankin, Director of Research at Bloodwise, said:
“Survival rates for childhood leukaemia have improved significantly and eight in 10 children will now survive in the long-term. Treatments are still highly toxic and can have devastating side effects and there is a need to improve survival further.
“Every child’s leukaemia is complex and genetically different, which can cause obstacles to the development of targeted treatments.
“Although this is early work, this study has identified common faults that play a role in driving the disease in the majority of children with this subtype of leukaemia, paving the way for future treatments.”