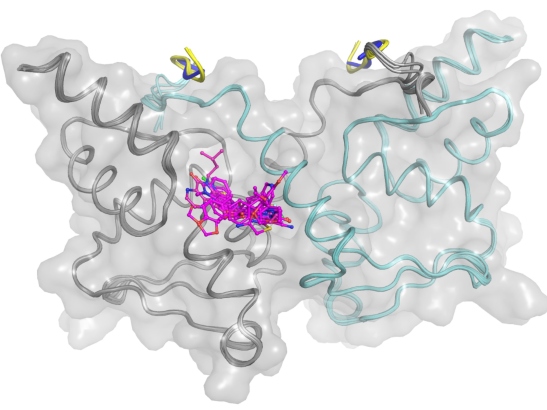
Image: the BCL6 BTB domain dimer (in grey/green) and compound hits in pink, superimposed on each other in the main compound binding site. Dr Rob van Montfort lab / ICR
Scientists have revealed details of the discovery of new inhibitors of the BCL6 protein, which is involved in driving several cancer types including the blood cancer B-cell lymphoma.
The inhibitors are small molecules that block BCL6’s action. With further research they could be developed into drugs to treat a range of cancer types, including blood cancers and solid tumours.
The compounds were discovered by researchers at The Institute of Cancer Research, London, through a multidisciplinary collaboration between assay scientists, medicinal chemists, biophysicists, structural biologists and other scientists at the Centre for Cancer Drug Discovery at the ICR.
In a new study published in the journal Scientific Reports, a team of scientists from the Centre for Cancer Drug Discovery describe an integrated workflow that led to the discovery of the novel inhibitors. The study was funded by Cancer Research UK, the Cancer Research Technology (CRT) Pioneer Fund and Sixth Element Capital LLP.
B-cell lymphoma 6 (BCL6) was initially discovered as a cancer-driving protein in B-cell lymphoma and has since been linked with an expanding number of blood cancers and solid tumours – including some leukaemias, breast cancer and non-small cell lung cancer (NSCLC).
Co-repressor proteins
The BCL6 protein acts by binding to hundreds of target genes, and then switching them off by recruiting several different ‘co-repressor’ proteins.
By effectively combining high throughput screening (HTS) with multiple biophysical techniques, X-ray crystallography and cell-based methods, the ICR researchers successfully discovered novel small molecule inhibitors that disrupt the interaction between the BCL6 protein and its corepressors.
These novel compounds offer the potential to block the ability of BCL6 to switch off its target genes and kill cancer cells.
State-of-the-art drug discovery
The search for BCL6 inhibitors began with the research team using high throughput screening, to identify compounds with activity against the protein-protein interactions of BCL6’s corepressor proteins. The team then used several different biophysical techniques to confirm these hits.
Using X-ray crystallography, the ICR team then determined the 3D structures of BCL6 bound to compounds from several different chemical series, enabling a structure-based drug design approach to improve their weak biochemical potency.
Confirming engagement
The next stage involved developing two different methods to determine the cellular activity of the improved inhibitors. The first was to confirm the inhibitors were engaging with their target zone on BCL6, and the second was to demonstrate and measure their inhibition of the interaction between BCL6 and its corepressor proteins.
Overall, the researchers’ comprehensive approach led to the discovery of novel inhibitors with respective biochemical and cellular potencies in the sub-micromolar and low-micromolar range – considered a robust starting point for further preclinical research to test their effectiveness in mice, and then if successful, on to clinical trials.
Study leader Dr Rob van Montfort, who leads the Hit Discovery and Structural Design Team at the ICR said:
“Identifying drug compounds that disrupt protein-protein interactions, such as the BCL6-corepressor interaction, has traditionally proved to be extremely challenging. Typically, high-throughput screening achieves low hit rates – and the weak potency of any genuine hits makes it difficult to distinguish them from false positives.
“By using a combination of biochemical and biophysical assays, followed by structural confirmation using X-ray crystallography, we were able to effectively discover several BCL inhibitors from multiple chemical series. It’s exciting to reach this stage, creating a shortlist of promising compounds and helping to improve them to the very potent and selective inhibitors we have published previously and that have the potential to move into the next phase of drug discovery.”