OPTIMISE-AR: Incorporating Patient-Reported Outcomes in Dose-Finding Trials - Analysis
In the OPTIMISE-AR project, we aim to identify core patient-reported outcomes (PRO) research objectives which should be considered in early phase dose finding oncology trials, and provide robust analysis recommendations for their use.
View the Participant Information Sheet with more details about the study. For additional information or help with any questions, please contact [email protected].
The primary goal of an early phase dose-finding trial is to evaluate the safety and tolerability of a new intervention and to determine the recommended dose(s) for further testing.
Patient-reported outcomes (PROs) can play a crucial role in this assessment by providing insights into the patient's quality of life, treatment tolerability, and functional status. Whilst existing PRO measures such as PRO-CTCAE are deemed suitable for patients to self-assess their treatment-related toxicities during a dose-finding trial, there are currently no guidelines on which PRO objectives should be assessed, nor on how to analyse or data visualise PROs data in early phase dose-finding trials.
We aim to develop recommendations through a modified Delphi survey approach involving multidisciplinary international participants. This guidance will enable trialists to benefit from the early insights that PROs can provide in EPDF trials while future evidence-based, best-practice recommendations continue to develop.
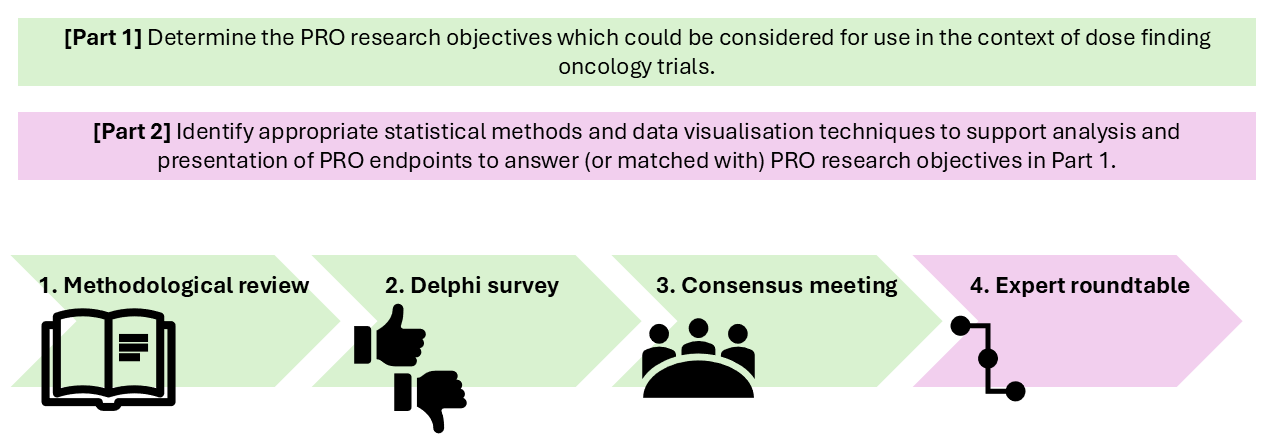
Step 1: Conduct methodological review and generate candidate items for PRO objectives.
Step 2: Evaluate candidate items with international multi-stakeholders in a two stage Delphi survey.
Step 3: Hold international consensus meeting to review Delphi survey findings.
Step 4: Match PRO objectives identified in the Delphi survey with associated analysis technique and data visualisation method during an expert roundtable involving statisticians, clinicians, regulators, journal editors, patient partners and PRO methodologists.
- [Part 1] Determine the PRO research objectives which could be considered for use in the context of dose finding oncology trials.
- [Part 2] Identify appropriate statistical methods and data visualisation techniques to support analysis and presentation of PRO endpoints to answer (or matched with) PRO research objectives in Part 1.
To ensure the robustness of methodology and subsequent recommendations, an international, multidisciplinary Executive Committee of specialists was assembled. This core team, comprising of statisticians, PRO methodologists, clinicians and patient advocates, meet routinely to provide continual feedback and discuss findings as the project develops.
- Emily Alger, Institute of Cancer Research (Co-Chief Investigator)
- Professor Christina Yap, Institute of Cancer Research (Co-Chief Investigator)
- Dr Lee Aiyegbusi, University of Birmingham
- Professor Ethan Basch, University of North Carolina at Chapel Hill
- Professor Mel Calvert, University of Birmingham
- Dr Amylou Dueck, Mayo Clinic
- Dr Anna Minchom, Institute of Cancer Research
- Dr Madeline Pe, European Organisation for Research and Treatment of Cancer
- Dr Devin Peipert, Northwestern University
- Professor Claire Snyder, Johns Hopkins University
- Dr Stefan Symeonides, University of Edinburgh
- Roger Wilson, Patient and Public Involvement lead