Our Research Programme
The overarching goal of our research programme is to identify and understand the molecular mechanisms behind immunogenic cell death so they can be manipulated in cancer therapies. Our research themes are (Figure 3):
Theme 1: Immunogenic cell death in cancer
• Identify regulatory mechanisms of necroptosis/apoptosis/pyroptosis and DAMPs production in the tumour
• Study the therapeutic consequences of immunogenic cell death on tumour/immune interactions in breast and lung cancer
Theme 2: Winners versus Losers: Cell Competition in tumour evolution, heterogeneity and drug resistance
• Develop novel anti-cancer treatment approaches that target the competitiveness features of the tumour microenvironment to convert super-competitor cancer cells into super-losers.
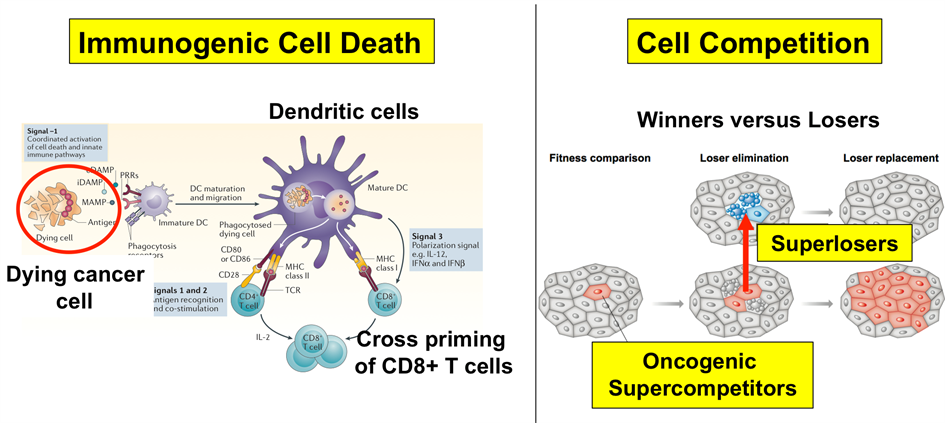
Figure 3: Research programme overview. Theme 1 (left): Immunogenic Cell Death (pushing current treatment regiments into immunogenic cell death). Theme 2 (right): Killing tumour cells by targeting the fitness landscape.
You can find more information in our research projects.
Our successes
• We established that IAPs (Inhibitors of APoptosis proteins) function at the crossroad of cell death regulation and inflammation; a view-point that is currently being exploited in clinical trials with pharmacological inhibitors of IAPs.
• We contributed to the characterisation of the ripoptosome, a large protein complex that can regulate cell death, mitosis, cell motility and cytokine production, with exciting implications for the design of novel therapeutic approaches aimed at selectively destroying apoptosis-resistant cancer cells.
• We reported the first pre-clinical application of an in vivo treatment protocol for soft-tissue sarcoma that directly engages RIPK1-mediated immunogenic cell death.
Our methods and techniques
Professor Meier and his group are using patient-derived organoids and animal models to investigate the complex relationship between cell death, immunity and tumorigenesis. Since these pathways are conserved between different species, it will be important to understand evolutionary principles that cause human diseases. Particularly, the laboratory is investigating the role of cell death, immunity and inflammation in adaptation to tissue stress, treatment resistance and tumour surveillance.