Project 1: Cell Death & Immunity
Background
There are multiple ways for a cell to die by cellular suicide. Living organisms have been fighting an evolutionary arms race against pathogens, and so cell death pathways have adapted and multiplied to cover the complexity of the foes the immune system faces.
Cell death is integral to the immune response and acts as a beacon, a second messenger, that alerts both the immune system and cells of the tissue micro-environment to ensure tissue repair and homeostasis. In our laboratory, we are interested in understanding the processes that regulate cell death, inflammation and immune homeostasis.
It is now clear that dying cancer cells play an active role in the initiation of an anti-cancer immune response. Cell death is accompanied by the release of constitutive and inducible DAMPs (damage associated molecular patterns), which act as drivers of the anti-tumour immune response.
However, different types of cell death have an unequal ability to trigger an immune response. Therefore, we have set out to understand the deep molecular and physiological nature of these cell death pathways, namely apoptosis, necroptosis and pyroptosis, and their relationship to both innate and adaptive immune systems.
Necroptosis and pyroptosis are known to be highly immunogenic, whereas apoptosis is thought to be mostly immunologically silent. Thus, although most current cancer treatment focus on apoptosis, the triggering of necroptosis and pyroptosis should be favoured in an anti-cancer therapeutic strategy.
Aim
We focus on the study of proteins that reside at the crossroads of cell death and immunity, such as IAPs, RIP kinases, PRRs (Pattern Recognition Receptors), MLKL and inflammasomes, which orchestrate the assembly of multimeric scaffolds that control cell activation, apoptosis, necroptosis and pyroptosis.
We aim to identify regulatory mechanisms of tumour cell necroptosis/apoptosis/pyroptosis and the production of inducible DAMPs, and study their consequence on tumour/immune interactions in breast and lung cancer therapy.
Approaches
Using mouse- and patient-derived organoids, we are studying the complex relationship between cell death and immunity. In collaboration with pharmaceutical companies we are designing innovative combinatory treatments to harness the power of immunogenic cell death as an anti-cancer therapy.
Expected outcome
Our work will help to steer current therapies towards the triggering of immunogenic cell death, and in combination with immune checkpoint blockade, lead to long-lasting anti-tumour immunity.
Project 2: Cell Competition
Background
Cancer treatment often fails because of the evolution of resistant clones. Tumour heterogeneity is a hallmark of many cancers, which generates an agglomeration of cells in which competitive interactions are able to occur among tumour cell subpopulations as well as neighbouring stromal tissue.
Here, we aim to harness the phenomenon of ‘cell competition’ to ‘steer’ cancer evolution in response to drugs, to control resistance and improve therapeutic outcomes. The ultimate goal is to restrain clonal evolution by changing the fitness landscape of cancer cells while strengthening the one of surrounding healthy tissue.
Cell competition is a cell fitness-sensing mechanism conserved from insects to mammals that eliminates those cells that, although viable, are less fit (Losers) than their neighbours (Winners) (Fig. 1).
An important implication of cell competition is that cellular fitness is not only a cell-intrinsic property but is also determined relative to the fitness of neighbouring cells: a cell that is of suboptimal fitness in one context may be ‘‘super-fit’’ in the context of a different cell population or drug exposure.
Strikingly, cancer cells, which are unhealthy, break that rule by acting as super-competitors to unfairly take control of the tissue.
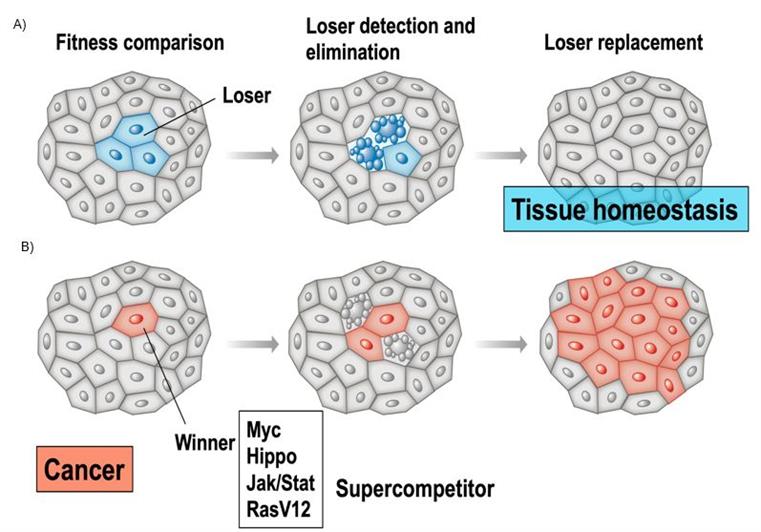
Figure 1: Cell competition in health (A) and disease (B).
(A) Less fit cells (blue) are eliminated by TNF-induced apoptosis (blebbing blue cells) and replaced by cells of the fitter type, ensuring tissue functionality and tissue homeostasis. (B) In the case of super-competition, super-competitor cancer cells (red) are able to outcompete wild-type cells (grey), inducing apoptosis of surrounding wild-type cells. Such WT cells are actively eliminated by competitive interactions.
Aim
We aim to identify how cells sense their relative fitness levels in the context of cell competition. We wish to develop novel anti-cancer treatment approaches that target the competitiveness of tumour cells, converting super-competitor tumour cells into super-losers.
Approaches
To delineate the mechanism of cell competition and track competing cells at single cell resolution, we are using animal models and a combination of state-of-the art imaging techniques and organoid cultures.
Expected outcome
Our work has highlighted that the acquisition of the super-competitor status of cancer cells comes at a cost, and that it is possible to tilt the balance to convert super-competitors into Losers. This process is intimately linked to metabolism and to the rewiring of cell death pathways, but the precise parameters governing this competition are poorly understood, especially in mammalians.
By killing super-competitor cancer cells, we aim to open a communication line with the immune system to promote anti-tumour immunity, offering a complementary approach to our strategies developed in Project 1.