One is an incurable childhood brain cancer, the other a strange and distressing inherited condition that gradually turns a patient’s muscle to bone. At first glance, the only connection between the two diseases is the devastating effects they have on patients and their families. But now, as covered in our news story, scientists at The Institute of Cancer Research, London, have uncovered the hidden genetic link between them, and opened up the prospect of an effective treatment for the childhood brain cancer for the first time.
A diagnosis of diffuse intrinsic pontine glioma (DIPG) is particularly cruel for children and their families, because this childhood brain cancer is essentially untreatable. The disease begins in the brain stem so surgery isn’t an option and it’s extremely difficult to deliver drugs to the affected region. But research by Dr Chris Jones – who leads the glioma team at the ICR – has now identified a key genetic driver for the disease. The findings from this study, which has mainly been funded by Cancer Research UK, has pointed researchers in the direction of potential treatments for the first time.
Dr Jones explains: “We found that a quarter of cases involve a mutatedACVR1 gene, which is incredibly promising because the gene provides a druggable target. If we can develop drugs to target this gene, we may finally be able to offer effective treatments for children with the disease.”
"This is the most exciting development in DIPG research since the disease was first diagnosed."
What’s fascinating about the ACVR1 gene is that it is not associated with any other cancers, but is implicated in "stone man syndrome", or Fibrodysplasia ossificans progressive (FOP). This is an extremely rare condition – only 600 cases reported worldwide – which causes muscles, tendons, ligaments and other connective tissues to turn to bone. Over decades, sufferers gradually become imprisoned within a ‘second skeleton’ and are unable to move. Eventually it becomes impossible to breathe, and most patients die before they reach 40. Children with FOP have the genetic flaw in all cells of their body because it is present in the fertilised egg form which they develop. These new results show that if the flaw occurs spontaneously in the brain, it could lead to DIPG.
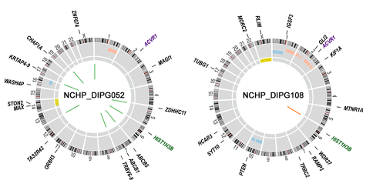
Genome analysis reveals the mutations behind a pair of DIPG cancers.
Having discovered that the two diseases can both be caused by ACVR1mutations, Dr Jones has been working with leading FOP researchers at the University of Oxford to explore treatments that could work for both diseases. It is extremely difficult to find new treatments for rare diseases, due to a lack of patients and funding, so Dr Jones hopes collaborations between researchers in both fields will be mutually beneficial.
One critical challenge in developing drugs for DIPG is that they must be able to cross the blood-brain barrier. The vast majority of cancer drugs do not need to enter the brain, and in fact for safety reasons it’s better that they don’t. However, as DIPG originates in the brain stem, it is absolutely vital that drugs can be delivered there. Dr Jones says: “As we’ve only just discovered that ACVR1 could provide a drug target in DIPG, it’s still early days. The Oxford FOP group have an ACVR1 inhibitor in the pipeline, but it’s yet to enter preclinical tests. But crucially, ACVR1 is found in a gene which produces a type of protein called a kinase – and there are many effective kinase inhibitors for different types of cancers out there, so we know this could be a potentially druggable target.”
In order to identify the gene in the first place, Dr Jones needed tumour tissue samples for genetic analysis. However, since the 1990s, it has been standard practice to diagnose DIPG based on symptoms and MRI scans rather than biopsies. This actually makes it more difficult to develop treatments, as Dr Jones explains: “Since people stopped performing brain biopsies around 20 years ago, we’ve had a bit of a ‘black hole’ in biopsy data. When there are no physical samples, we lose the ability to perform advanced tests such as genetic sequencing. Fortunately, in 2007 a Parisian neurosurgeon developed a safe method of biopsying brain tumours in children. Working with parents and patient groups, we were able to use 21 samples from such biopsies as well as five from autopsies to perform genetic sequencing. This allowed us to locate the gene mutation, which could end up saving many lives as treatments are developed in years to come.”
New therapies are desperately needed for DIPG, and Dr Jones’ research looks set to lead the way in developing new targeted drugs for this devastating and incurable childhood cancer.