Precision Oncology Group
Professors Chris Lord and Andrew Tutt’s group investigates the molecular basis of cancer to better understand and treat the disease.
Research, projects and publications in this group
We have a multidisciplinary approach to our work - our group is made of cell biologists, geneticists, biochemists, in vivo specialists, bioinformaticians and oncologists. Together we aim to understanding how to exploit the molecular changes that occur in tumours to develop new treatments or to improve existing treatments.
Although tumour cells gain a series of characteristics that provide a selective advantage, the molecular events that drive these processes also impart upon the cell a series of dependencies. Our work focusses on identifying and understanding these tumour specific dependencies, such as synthetic lethal effects. We also acknowledge that cancers evolve, especially in the face of the selective pressure of treatment. Because of this, we are also interested in how therapeutic resistance in cancer occurs and how this might be targeted.
Unusually, we are a joint laboratory being led by both a biologist and a clinician scientist. Together with our colleague Stephen Pettitt we unashamedly focus our Precision Oncology laboratory work on delivering solutions to real-world clinical problems that people with cancer face. This means we focus on generating information which could either: (i) inform the design or interpretation of clinical trials in cancer; (ii) inform the identity of biomarkers that identify how to most effectively treat each cancer patient; and (iii) identify novel drug targets and therapeutic approaches to treating cancer.
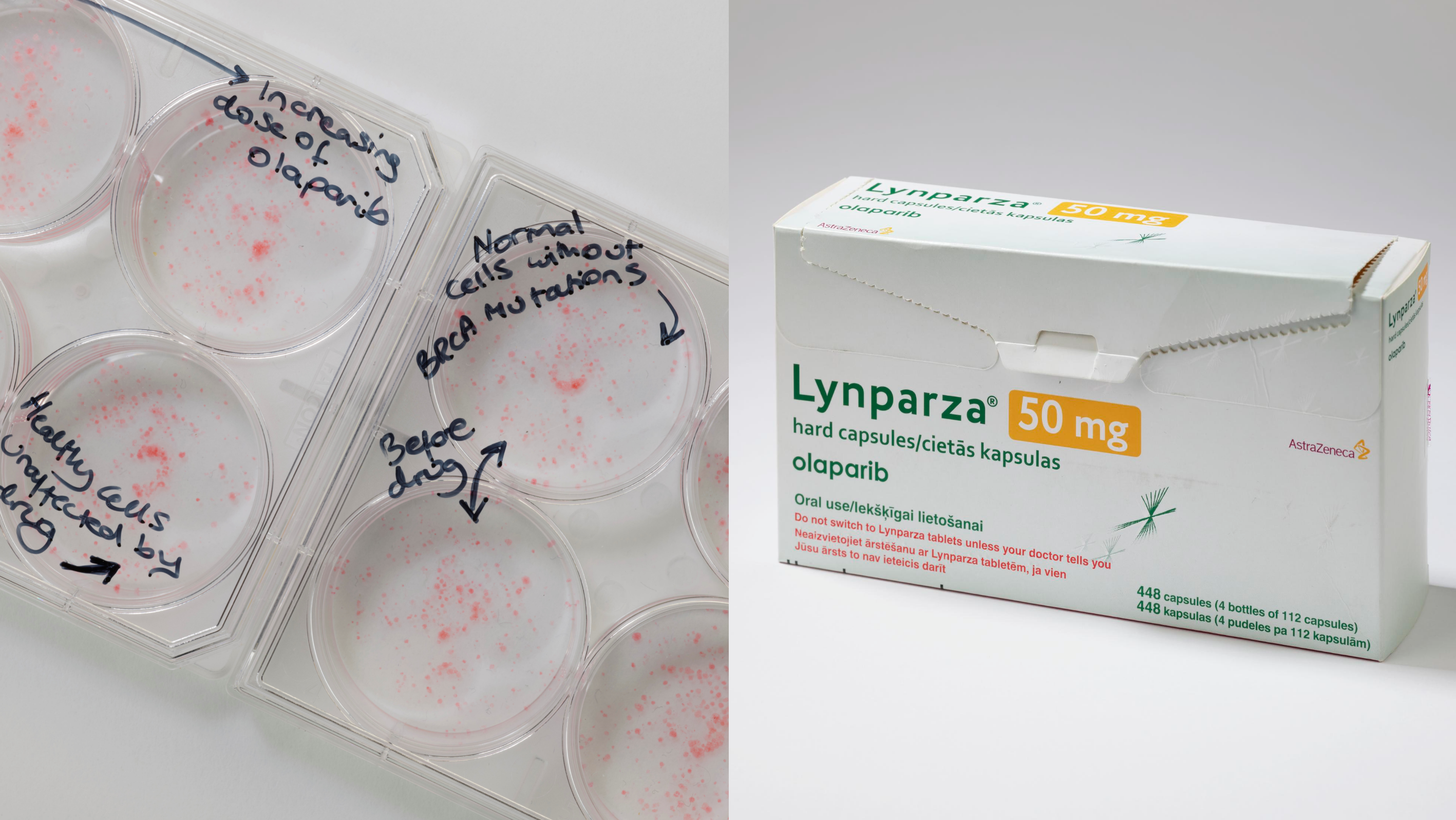
To this end, we have made major advances in identifying novel synthetic lethal interactions that have translational potential, the most notable being the identification of synthetic lethality between PARP inhibitors and genes involved in the homologous recombination (HR) pathway such as BRCA1 and BRCA2. We, with Alan Ashworth, not only carried out some of the first pre-clinical work that led to the use of PARP inhibitors in clinical trials but also designed and led some of the key clinical trials that resulted in the approval of these drugs in HR deficient cancers. Alongside this work, we have also spent the last 20 years studying how PARP inhibitor resistance occurs and how this might be targeted. This still remains an active part of our research interests.
Using the same concept of targeting tumour-specific dependencies, we have also made advances in systematically identifying additional synthetic lethal effects that operate in breast and other cancers that have alterations in or expression of cancer-associated genes such as E-cadherin, HORMAD1 and Rb. In each case, we are focussed on either identifying drug targets that could inform new drug discovery programmes or identifying existing drugs that could be repurposed for use in biomarker-defined cancer populations.
We are truly multidisciplinary in our approach, as one would expect from a laboratory led by a biologist and a clinician scientist. For example, we commonly integrate information from in vitro and in vivo genetic perturbation approaches with the analysis of patient-derived material. This use of both forward and reverse translation approaches ensures that the discoveries we make are relevant to the clinical problems we are trying to solve. Because of this, our laboratory is truly a multidisciplinary and international team, including cell biologists, geneticists, biochemists, in vivo specialists, computational biologists and oncologists, all of whom work towards the same aims.
We are always interested in hearing from people who want to work in this area, especially from those interested in carrying out post-doctoral research in the area of precision medicine.
- The transcriptomic architecture of common cancers reflects synthetic lethal interactions. Haider S, Brough R, Madera S, Iacovacci J, Gulati A, Wicks A, Alexander J, Pettitt SJ, Tutt ANJ, Lord CJ. Nat Genetics (2025) 57(3):522-529.
- Longitudinal profiling identifies co-occurring BRCA1/2 reversions, TP53BP1, RIF1 and PAXIP1 mutations in PARP inhibitor resistant advanced breast cancer. Harvey-Jones E, Raghunandan M, Robbez-Masson L, Magraner-Pardo L, Alaguthurai T, Yablonovitch A, Yen J, Xiao H, Brough R, Frankum J, Song F, Yeung J, Savy T, Gulati A, Alexander J, Kemp H, Starling C, Konde A, Marlow R, Cheang M, Proszek P, Hubank M, Cai M, Trendell J, Lu R, Liccardo R, Ravindran N, Llop-Guevara A, Rodriguez O, Balmana J, Lukashchuk N, Dorschner M, Drusbosky L, Roxanis I, Serra V, Haider S, Pettitt SJ, Lord CJ, Tutt ANJ. Ann Oncol. (2024) 35(4):364-380.
- Complex synthetic lethality in cancer. Ryan CJ, Devakumar LPS, Pettitt SJ, Lord CJ. Nature Genetics (2023) 55(12):2039-2048.
- Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Geyer CE Jr, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, Rastogi P, Cui K, Arahmani A, Aktan G, Armstrong AC, Arnedos M, Balmaña J, Bergh J, Bliss J, Delaloge S, Domchek SM, Eisen A, Elsafy F, Fein LE, Fielding A, Ford JM, Friedman S, Gelmon KA, Gianni L, Gnant M, Hollingsworth SJ, Im SA, Jager A, Jóhannsson ÓÞ, Lakhani SR, Janni W, Linderholm B, Liu TW, Loman N, Korde L, Loibl S, Lucas PC, Marmé F, Martinez de Dueñas E, McConnell R, Phillips KA, Piccart M, Rossi G, Schmutzler R, Senkus E, Shao Z, Sharma P, Singer CF, Španić T, Stickeler E, Toi M, Traina TA, Viale G, Zoppoli G, Park YH, Yerushalmi R, Yang H, Pang D, Jung KH, Mailliez A, Fan Z, Tennevet I, Zhang J, Nagy T, Sonke GS, Sun Q, Parton M, Colleoni MA, Schmidt M, Brufsky AM, Razaq W, Kaufman B, Cameron D, Campbell C, Tutt ANJ; OlympiA Clinical Trial Steering Committee and Investigators. Ann Oncol. 2022 33(12):1250-1268.
- The ubiquitin-dependent ATPase p97 removes cytotoxic trapped PARP1 from chromatin. Krastev DB, Li S, Sun Y, Wicks AJ, Hoslett G, Weekes D, Badder LM, Knight EG, Marlow R, Pardo MC, Yu L, Talele TT, Bartek J, Choudhary JS, Pommier Y, Pettitt SJ, Tutt ANJ, Ramadan K, Lord CJ. Nat Cell Biol. 24(1):62-73 (2022)
- Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance. Zatreanu D, Robinson HMR, Alkhatib O, Boursier M, Finch H, Geo L, Grande D, Grinkevich V, Heald RA, Langdon S, Majithiya J, McWhirter C, Martin NMB, Moore S, Neves J, Rajendra E, Ranzani M, Schaedler T, Stockley M, Wiggins K, Brough R, Sridhar S, Gulati A, Shao N, Badder LM, Novo D, Knight EG, Marlow R, Haider S, Callen E, Hewitt G, Schimmel J, Prevo R, Alli C, Ferdinand A, Bell C, Blencowe P, Bot C, Calder M, Charles M, Curry J, Ekwuru T, Ewings K, Krajewski W, MacDonald E, McCarron H, Pang L, Pedder C, Rigoreau L, Swarbrick M, Wheatley E, Willis S, Wong AC, Nussenzweig A, Tijsterman M, Tutt A, Boulton SJ, Higgins GS, Pettitt SJ, Smith GCM, Lord CJ. Nat Comms. 12(1):3636 (2021)
- Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, Gelber RD, de Azambuja E, Fielding A, Balmaña J, Domchek SM, Gelmon KA, Hollingsworth SJ, Korde LA, Linderholm B, Bandos H, Senkus E, Suga JM, Shao Z, Pippas AW, Nowecki Z, Huzarski T, Ganz PA, Lucas PC, Baker N, Loibl S, McConnell R, Piccart M, Schmutzler R, Steger GG, Costantino JP, Arahmani A, Wolmark N, McFadden E, Karantza V, Lakhani SR, Yothers G, Campbell C, Geyer CE Jr; OlympiA Clinical Trial Steering Committee and Investigators. N Engl J Med. (2021) 384(25):2394-2405
- Clinical BRCA1/2 Reversion Analysis Identifies Hotspot Mutations and Predicted Neoantigens Associated with Therapy Resistance. Pettitt SP, Frankum JR, Punta M, Lise S, Alexander J, Chen Y, Yap TA, Haider S, Tutt NJ, Lord CJ. Cancer Discov. 1475-1488 (2020)
- Integrative analysis of large-scale loss-of-function screens identifies robust cancer-associated genetic interactions. Lord CJ, Quinn N, Ryan CJ. Elife. 9. pii: e58925 (2020)
- E-Cadherin/ROS1 Inhibitor Synthetic Lethality in Breast Cancer. Bajrami I, Marlow R, van de Ven M, Brough R, Pemberton HN, Frankum J, Song F, Rafiq R, Konde A, Krastev DB, Menon M, Campbell J, Gulati A, Kumar R, Pettitt SJ, Gurden MD, Cardenosa ML, Chong I, Gazinska P, Wallberg F, Sawyer EJ, Martin LA, Dowsett M, Linardopoulos S, Natrajan R, Ryan CJ, Derksen PWB, Jonkers J, Tutt ANJ, Ashworth A, Lord CJ. Cancer Discov. 8(4):498-515. (2018)
- Genome-wide and high-density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Pettitt SJ, Krastev DB, Brandsma I, Dréan A, Song F, Aleksandrov R, Harrell MI, Menon M, Brough R, Campbell J, Frankum J, Ranes M, Pemberton HN, Rafiq R, Fenwick K, Swain A, Guettler S, Lee JM, Swisher EM, Stoynov S, Yusa K, Ashworth A, Lord CJ. Nat Commun. 10;9(1):1849. (2018)
- Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Tutt, A., H. Tovey, M. C. U. Cheang, S. Kernaghan, L. Kilburn, P. Gazinska, J. Owen, J. Abraham, S. Barrett, P. Barrett-Lee, R. Brown, S. Chan, M. Dowsett, J. M. Flanagan, L. Fox, A. Grigoriadis, A. Gutin, C. Harper-Wynne, M. Q. Hatton, K. A. Hoadley, J. Parikh, P. Parker, C. M. Perou, R. Roylance, V. Shah, A. Shaw, I. E. Smith, K. M. Timms, A. M. Wardley, G. Wilson, C. Gillett, J. S. Lanchbury, A. Ashworth, N. Rahman, M. Harries, P. Ellis, S. E. Pinder, and J. M. Bliss. Nat Med (2018) 24: 628-37.
- Genomic Complexity Profiling Reveals That HORMAD1 Overexpression Contributes to Homologous Recombination Deficiency in Triple-Negative Breast Cancers. Watkins J, Weekes D, Shah V, Gazinska P, Joshi S, Sidhu B, Gillett C, Pinder S, Vanoli F, Jasin M, Mayrhofer M, Isaksson A, Cheang MC, Mirza H, Frankum J, Lord CJ, Ashworth A, Vinayak S, Ford JM, Telli ML, Grigoriadis A, Tutt AN. Cancer Discov. (2015) 5(5):488-505.
- PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Brasó-Maristany F, Filosto S, Catchpole S, Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L, Gazinska P, Liccardi G, Meier P, Gris-Oliver A, Cheang MC, Perdrix-Rosell A, Shafat M, Noël E, Patel N, McEachern K, Scaltriti M, Castel P, Noor F, Buus R, Mathew S, Watkins J, Serra V, Marra P, Grigoriadis A, Tutt AN. Nat Med. (2016) 22(11):1303-1313.
- Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Audeh, M. W., J. Carmichael, R. T. Penson, M. Friedlander, B. Powell, K. M. Bell-McGuinn, C. Scott, J. N. Weitzel, A. Oaknin, N. Loman, K. Lu, R. K. Schmutzler, U. Matulonis, M. Wickens, and A. Tutt. Lancet (2010) 376: 245-51
- Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Tutt, A., M. Robson, J. E. Garber, S. M. Domchek, M. W. Audeh, J. N. Weitzel, M. Friedlander, B. Arun, N. Loman, R. K. Schmutzler, A. Wardley, G. Mitchell, H. Earl, M. Wickens, and J. Carmichael. Lancet (2010) 376: 235-44.
- Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Farmer, H., McCabe, N., Lord, C.J., Tutt, A. N., Johnson, D. A., Richardson, T. B., Santarosa, M., Dillon, K. J., Hickson, I., Knights, C., Martin, N. M., Jackson, S. P., Smith, G. C. & Ashworth, A. Nature 434, 917-921, (2005).
If you are interested in coming to work with us, please send your CV and a cover letter (explaining your research interests) to [email protected], [email protected] and [email protected].
Researchers in this group
Professor Andrew Tutt
Director of Breast Cancer Now Toby Robins Research Centre, Head of Division & Group Leader:
Precision Oncology
Andrew Tutt is Head of the Division of Breast Cancer Research and Director of the Breast Cancer Now Toby Robins Research Centre at the ICR and Guy’s Hospital King’s College London. He is a Clinician Scientist with the Laboratory and Clinical Trials programme, and a Consultant Clinical Oncologist looking after women with breast cancer.
Professor Chris Lord
Deputy Head of Division & Group Leader:
Precision Oncology.tmb-propic-md.png?Culture=en&sfvrsn=a7fc4e90_9)
Professor Chris Lord is Deputy Head of Division and the leader of the CRUK Gene Function Group, which applies concepts such as synthetic lethality and non-oncogene addiction to provide one route to identifying novel approaches to treating cancer.
 .
.
Dr Dragomir Krastev is a postdoctoral fellow who specialises in studying the mechanisms and regulation of a type of post-translational protein modification called PARsylation.
 .
.
Dr Steve Pettitt is a Senior Staff Scientist in the Gene Function group. He also is recipient of a Dean’s Pathways to Independence Award.
 .
.
Andrew Tutt is Head of the Division of Breast Cancer Research and Director of the Breast Cancer Now Toby Robins Research Centre at the ICR and Guy’s Hospital King’s College London. He is a Clinician Scientist with the Laboratory and Clinical Trials programme, and a Consultant Clinical Oncologist looking after women with breast cancer.
.png?sfvrsn=a7fc4e90_2) .
.
Professor Chris Lord is Deputy Head of Division and the leader of the CRUK Gene Function Group, which applies concepts such as synthetic lethality and non-oncogene addiction to provide one route to identifying novel approaches to treating cancer.
Industrial partnership opportunities with this group
Opportunity: Cancer biomarker for predicting response to drugs targeting mitotic checkpoint kinases and cell division
Commissioner: Professor Andrew Tutt, Professor Chris Lord, Professor Jonathon Pines
Opportunity: ARID1A and other BAF complex defects as biomarkers for ATRi resistance
Commissioner: Professor Chris Lord
-carousel-945x581.jpg?sfvrsn=d29fb9e9_2)
.jpg?sfvrsn=b8be1e0b_2)