Image: Graphic depicting protein degradation. Credit: CS Chem. Biol. 2024, 19, 1, 173-184
Researchers have discovered ways to convert inhibitor-style targeted cancer drugs into small molecules known as degraders, which help destroy cancer-promoting proteins in cells.
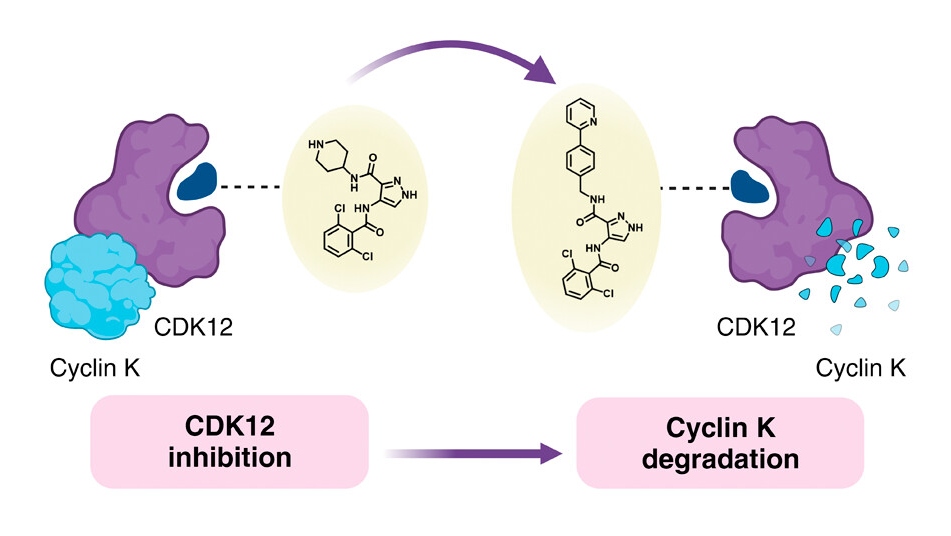
The scientists selected molecules known to inhibit certain proteins – stopping their function – and modified them in various ways to convert them into protein degraders, which break down the protein.
Other scientists can now replicate this novel approach to develop and optimise a range of protein degraders. In the long term, this may help with making existing cancer treatments more effective and creating new treatments to target different proteins involved in cancer, including proteins that may previously have been considered ‘undruggable’.
This innovative work, which was led by researchers at The Institute of Cancer Research, London, was published in ACS Chemical Biology. It was funded by The Institute of Cancer Research (ICR), which is both a research institute and a charity, and by a PhD Studentship from the Medical Research Council and AstraZeneca.
The need to optimise targeted protein degradation
Scientists worldwide have been investigating targeted protein degradation as a way to eliminate specific proteins known to play a part in the development or progression of cancer. Cells naturally break down damaged or unnecessary proteins, and it is possible to hijack this process by introducing the right drug molecules so that the cells destroy cancer proteins.
The ICR is committed to researching targeted protein degradation as a way to discover new cancer treatments. In 2022, a major philanthropic donation made it possible to open a new Centre for Protein Degradation within the ICR’s Centre for Cancer Drug Discovery.
Researchers have already achieved some success using proteolysis targeting chimeras (PROTACs). These bind to both the protein of interest and an enzyme called an E3 ubiquitin ligase to bring the two close together. The enzyme then labels the protein with ubiquitin, which marks it for destruction by the cell.
However, the relatively high molecular weight of PROTACs limits their use, as they do not dissolve well in water and can struggle to enter cells in a high enough concentration to be effective. Conversely, if the concentration becomes too high, the PROTACs all bind to only one of the two compounds – the enzyme or the target protein – rather than both, meaning protein degradation is incomplete.
A more recent discovery has been the use of monovalent degraders, often referred to as molecular glues. These work in a similar way to PROTACs, but they chemically modify the surface of either the enzyme or the target protein so that the two can directly bind. These compounds overcome the limitations of PROTACs because they are smaller and still effective at higher concentrations.
A molecular glue drug, discovered following a programme of research at the ICR, is already in early phase clinical trials.
Turning inhibitors into degraders
Wanting to understand the link between the chemical structure of molecular glues and how they behave – known as the structure-activity relationship (SAR) – the researchers behind the current study decided to use a known monovalent degrader called CR8 as a starting point. CR8 inhibits all cyclin-dependent kinases (CDKs), which have multiple roles in tumour development, but it also degrades the protein cyclin K, which promotes cancer signalling pathways.
Previous research has shown that CR8 binds to CDK12 and an E3 ligase component called damaged DNA binding protein 1 (DDB1). This allows the cell to break down cyclin K bound to CDK12.
Armed with this information, the team tried replacing the pyridine in CR8 with a broad range of substitutes. They first tried using simple groups, including fluorine, methyl and hydroxy, before moving on to more complex formations to determine how these changes affected degradation ability.
In the second part of the study, the researchers applied their increased understanding of degrader SAR to other CDK-inhibitors to determine whether their findings were transferable. They found that molecular components that induced protein degradation could be used to convert multiple different inhibitors into degraders. They also identified certain substitutes that led to improved degradation.
First author Katie Thomas, a PhD student in the Division of Cancer Therapeutics at the ICR, said:
“It was surprising to see that such a wide range of groups could be used to induce cyclin K degradation. This means that the interaction interface between degrader bound-CDK12 and DDB1 does not need to be perfectly optimised for degradation to occur. This is promising for the future of using monovalent glues as therapeutic modalities – it will help us progress molecules from discovery through to optimisation.”
Degraders are more effective than inhibitors
Protein kinase inhibitors have been used in cancer treatment for more than two decades. They work by blocking the action of protein kinases, enzymes that control the function of proteins and can lead to unregulated cell growth if they become mutated.
Although these treatments can work well, there is a risk of the cancer becoming resistant to them. This is because inhibitors obstruct cancer-related proteins rather than destroying them, and the tumour cells can sometimes reactivate them.
In addition, when some protein kinases are blocked by inhibitors, the cell compensates by using other kinases to perform the same actions.
Getting rid of the proteins entirely through protein degradation removes these risks.
A foundation for further work
This study serves as a starting point for other researchers who are looking to design molecular glues, identify promising protein targets for degradation and develop new molecules that can function as degrader therapies.
Thomas said:
“We hypothesise that other small molecule inhibitors could be converted into degraders through the addition of certain groups. Our team is currently exploring whether certain groups can be joined to other types of kinase inhibitors to turn them into monovalent degraders. Work is also underway to determine whether these compounds can degrade other proteins.”
In theory, once researchers have gained a full understanding of degrader SAR, it will be possible to remove a range of cancer-promoting proteins across different types of cancer. This approach could, therefore, open the door to new effective cancer treatments.
Corresponding author Dr Benjamin Bellenie, Senior Staff Scientist in the Division of Cancer Therapeutics at the ICR, said:
“This study has significantly improved our understanding around the design of monovalent degraders. We have focused specifically on cyclin K degraders and need to work out whether we can replicate this approach for new targets. However, we believe that our research could enable future drug discoveries.”