Reducing mouse numbers by measuring tumour size non-invasively
Scientists at the ICR are increasingly using non-invasive methods to measure the size of tumours in mice – allowing the same mouse to be assessed several times and reducing the overall numbers needed in our research.
In studies of new cancer treatments, researchers test whether a potential drug can shrink a tumour or slow its growth. An important step is often to assess a new drug in mice, to see if it is delivered where needed in the body and whether it has therapeutic benefit. To gather data effectively, it is important that scientists can accurately measure the size of tumours in mice to see whether the treatment being tested is having any effect, and also assess any ‘off-target’ effects on normal tissues.
In some studies we may implant cancer cells derived from patients into a mouse organ, while in others we use genetically engineered mice that develop tumours spontaneously. Studies of cancer in mice can mimic the complex way tumours grow and spread in people with cancer. But it can be hard to accurately measure a tumour and how it is being affected by treatment without removing it from the mouse’s body.
Dr Simon Robinson and his team at the ICR are using magnetic resonance imaging (MRI), commonly used for patients in the clinic, to measure tumours in mice. MRI uses a magnetic field and non-ionising radiation to provide us with images of tumours and other tissues inside of the body. The technique is non-invasive, allowing researchers to assess the same mouse on more than one occasion, and to make comparisons before and after treatment. This gives us enough information to use statistical tests that give us valid results with a smaller total number of mice used. In one study using MRI in genetically engineered mice, ICR researchers obtained meaningful data about the effects of a drug from just 12 mice, compared with the 32 that would have been needed otherwise.
Dr Simon Robinson, Team Leader in Magnetic Resonance at the ICR, said: “Animal welfare and ways of reducing animal use in drug discovery, development and safety are important areas for researchers. Using imaging techniques such as MRI to non-invasively monitor tumour response in mice is a powerful research approach which we can use to accurately evaluate new drugs in smaller numbers of animals. Such studies can also accelerate the clinical development of a treatment, or bring about early closure of a project unlikely to produce a useful drug, saving both time and money.”
Using 3D cell models to screen out ineffective agents before animal studies
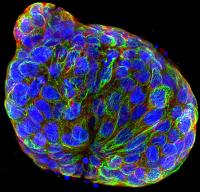
Scientists at the ICR are growing spheres of cancer cells in the laboratory which are designed to mimic the properties of solid tumours. Testing new compounds against these ‘micro-cancers’ can give a better idea of whether a treatment is likely to work than using conventional flat cell models – and could significantly reduce the number that go through to studies in mice.
Researchers first test new agents in the laboratory to assess their effects on cancer cell growth, selecting the best for animal studies. But standard two-dimensional single layers of tumour cells grown in plastic dishes cannot mimic the complex microenvironment of tumours within the body. These 2D cultures fail to recreate complex features of solid tumours such as variability in cell behaviour and differences in drug access to surface and deeper regions. Neither do they address important characteristics of malignant cells such as migration and invasion.
A team led by Professor Sue Eccles and supported by the NC3Rs has developed a new cell culture system which more closely replicates the structures and behaviours of real cancers. The researchers designed a method to grow 96 identical micro-cancers in a single plate the size of a postcard. These micro-cancers show features of solid tumours such as limited access to oxygen and nutrients in central areas. The researchers use use new imaging technologies to continuously monitor cancer cell growth and response to drugs, and importantly their ability to move and invade tissues.
The group initially focused on brain cancers which, because of their invasive nature, are often fatal in both adults and children and novel therapies are urgently needed. The researchers have also extended the assays to other common cancers such as breast, lung, bowel and prostate.
By using cell culture models which more closely mimic human tumours, scientists can narrow down their search for new drugs to just the most promising candidates. In just one project at the ICR the team selected 30% fewer compounds to progress to testing in mice.
The 3D cell models can be used to screen many potential new cancer drugs at once, and will be especially valuable for identifying agents active against advanced, metastatic disease. The team estimates that if rolled out across the sector, it could reduce the number of mice used by the pharmaceutical industry in the UK by around 40,000 each year.
Professor Sue Eccles, Professor of Experimental Cancer Therapeutics said: “As a member of the ICR’s Animal Welfare and Ethics Review Board, I am fully committed to developing alternatives to animal use wherever possible. While it is mandatory to test new agents in animals to show that they are safe and likely to be effective in patients, I believe that these advanced but easy-to-use 3D assays will be more predictive than standard 2D cell cultures, and will allow us to reduce significantly the number of animals needed to bring each cancer drug to the clinic.”
Further information
Vinci et al. (2012) Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation BMC Biology, 10:29.
Zimmermann et al. (2013) Two-dimensional vs three-dimensional in vitro tumor migration and invasion assays. Methods Mol Biol, 986:227-252.
Vinci et al. (2013) Tumor spheroid-based migration assays for evaluation of therapeutic agents. Methods Mol Biol, 253-266.
Box et al. (2013) A progression-related protein signature in head and neck squamous cell carcinoma associated with acquired resistance to multiple EGFR tyrosine kinase inhibitors. Eur J Cancer, 2512-21.
A video showing how to set up invasion assays: Vinci M, Box C, Eccles S. Three-dimensional (3D) tumor spheroid invasion assay.